The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells
- PMID: 19670250
- PMCID: PMC2933782
- DOI: 10.1002/cmmi.276
The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells
Abstract
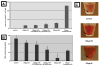
For in vivo applications of magnetically labeled stem cells, biological effects of the labeling procedure have to be precluded. This study evaluates the effect of different ferucarbotran cell labeling protocols on chondrogenic differentiation of human mesenchymal stem cells (hMSC) as well as their implications for MR imaging. hMSC were labeled with ferucarbotran using various protocols: cells were labeled with 100 microg Fe/ml for 4 and 18 h and additional samples were cultured for 6 or 12 days after the 18 h labeling. Supplementary samples were labeled by transfection with protamine sulfate. Iron uptake was quantified by ICP-spectrometry and labeled cells were investigated by transmission electron microscopy and by immunostaining for ferucarbotran. The differentiation potential of labeled cells was compared with unlabeled controls by staining with Alcian blue and Hematoxylin and Eosin, then quantified by measurements of glucosaminoglycans (GAG). Contrast agent effect at 3 T was investigated on days 1 and 14 of chondrogenic differentiation by measuring signal-to-noise ratios on T(2)-SE and T(2)*-GE sequences. Iron uptake was significant for all labeling protocols (p < 0.05). The uptake was highest after transfection with protamine sulfate (25.65 +/- 3.96 pg/cell) and lowest at an incubation time of 4 h without transfection (3.21 +/- 0.21 pg/cell). While chondrogenic differentiation was decreased using all labeling protocols, the decrease in GAG synthesis was not significant after labeling for 4 h without transfection. After labeling by simple incubation, chondrogenesis was found to be dose-dependent. MR imaging showed markedly lower SNR values of all labeled cells compared with the unlabeled controls. This contrast agent effect persisted for 14 days and the duration of differentiation. Magnetic labeling of hMSC with ferucarbotran inhibits chondrogenesis in a dose-dependent manner when using simple incubation techniques. When decreasing the incubation time to 4 h, inhibition of chondrogenesis was not significant.
Copyright (c) 2009 John Wiley & Sons, Ltd.
Figures


Similar articles
-
Labeling human mesenchymal stem cells with fluorescent contrast agents: the biological impact.Mol Imaging Biol. 2011 Feb;13(1):3-9. doi: 10.1007/s11307-010-0322-0. Mol Imaging Biol. 2011. PMID: 20379785 Free PMC article.
-
Direct labeling of hMSC with SPIO: the long-term influence on toxicity, chondrogenic differentiation capacity, and intracellular distribution.Mol Imaging Biol. 2011 Jun;13(3):443-451. doi: 10.1007/s11307-010-0360-7. Mol Imaging Biol. 2011. PMID: 20567925
-
Bifunctional Labeling of Rabbit Mesenchymal Stem Cells for MR Imaging and Fluorescence Microscopy.Mol Imaging Biol. 2020 Apr;22(2):303-312. doi: 10.1007/s11307-019-01385-8. Mol Imaging Biol. 2020. PMID: 31209781
-
Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis.NMR Biomed. 2004 Nov;17(7):513-7. doi: 10.1002/nbm.925. NMR Biomed. 2004. PMID: 15526348
-
Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells.NMR Biomed. 2005 Dec;18(8):553-9. doi: 10.1002/nbm.991. NMR Biomed. 2005. PMID: 16229060
Cited by
-
In Vivo Cellular Magnetic Imaging: Labeled vs. Unlabeled Cells.Adv Funct Mater. 2022 Dec 9;32(50):2207626. doi: 10.1002/adfm.202207626. Epub 2022 Aug 22. Adv Funct Mater. 2022. PMID: 36589903 Free PMC article.
-
Stem cell tracking using iron oxide nanoparticles.Int J Nanomedicine. 2014 Mar 31;9:1641-53. doi: 10.2147/IJN.S48979. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24729700 Free PMC article. Review.
-
Hyperthermia treatment of tumors by mesenchymal stem cell-delivered superparamagnetic iron oxide nanoparticles.Int J Nanomedicine. 2016 May 9;11:1973-83. doi: 10.2147/IJN.S94255. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27274229 Free PMC article.
-
MR signal characteristics of viable and apoptotic human mesenchymal stem cells in matrix-associated stem cell implants for treatment of osteoarthritis.Invest Radiol. 2010 Oct;45(10):634-40. doi: 10.1097/RLI.0b013e3181ed566c. Invest Radiol. 2010. PMID: 20808236 Free PMC article.
-
In vivo magnetic resonance imaging and optical imaging comparison of viable and nonviable mesenchymal stem cells with a bifunctional label.Mol Imaging. 2010 Oct;9(5):278-90. Mol Imaging. 2010. PMID: 20868628 Free PMC article.
References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. - PubMed
-
- Matsubara T, Tsutsumi S, Pan H, Hiraoka H, Oda R, Nishimura M, Kawaguchi H, Nakamura K, Kato Y. A new technique to expand human mesenchymal stem cells using basement membrane extracellular matrix. Biochem Biophys Res Commun. 2004;313:503–508. - PubMed
-
- Curran JM, Chen R, Hunt JA. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials. 2006;27:4783–4793. - PubMed
-
- Mastrogiacomo M, Muraglia A, Komlev V, Peyrin F, Rustichelli F, Crovace A, Cancedda R. Tissue engineering of bone: search for a better scaffold. Orthod Craniofac Res. 2005;8:277–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
