Hypertrophic gene expression induced by chronic stretch of excised mouse heart muscle
- PMID: 19670825
- PMCID: PMC3272304
Hypertrophic gene expression induced by chronic stretch of excised mouse heart muscle
Abstract
Altered mechanical stress and strain in cardiac myocytes induce modifications in gene expression that affects cardiac remodeling and myocyte contractile function. To study the mechanisms of mechanotransduction in cardiomyocytes, probing alterations in mechanics and gene expression has been an effective strategy. However, previous studies are self-limited due to the general use of isolated neonatal rodent myocytes or intact animals. The main goal of this study was to develop a novel tissue culture chamber system for mouse myocardium that facilitates loading of cardiac tissue, while measuring tissue stress and deformation within a physiological environment. Intact mouse right ventricular papillary muscles were cultured in controlled conditions with superfusate at 95% O2/ 5% CO2, and 34 degrees C, such that cell to extracellular matrix adhesions as well as cell to cell adhesions were undisturbed and both passive and active mechanical properties were maintained without significant changes. The system was able to measure the induction of hypertrophic markers (BNP, ANP) in tissue after 2 hrs and 5 hrs of stretch. ANP induction was highly correlated with the diastolic load of the muscle but not with developed systolic load. Load induced ANP expression was blunted in muscles from muscle-LIM protein knockout mice, in which defective mechanotransduction pathways have been predicted.
Figures
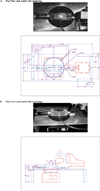

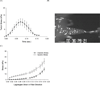
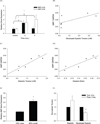
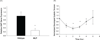
Similar articles
-
Effects of stretch and shortening on gene expression in intact myocardium.Physiol Genomics. 2014 Jan 15;46(2):57-65. doi: 10.1152/physiolgenomics.00103.2013. Epub 2013 Dec 3. Physiol Genomics. 2014. PMID: 24302644 Free PMC article.
-
Overexpression of miR-142-3p improves mitochondrial function in cardiac hypertrophy.Biomed Pharmacother. 2018 Dec;108:1347-1356. doi: 10.1016/j.biopha.2018.09.146. Epub 2018 Oct 4. Biomed Pharmacother. 2018. PMID: 30372837
-
Gene expression of atrial natriuretic peptide in rat papillary muscle. Rapid induction by mechanical loading.FEBS Lett. 1994 Jun 13;346(2-3):185-8. doi: 10.1016/0014-5793(94)00464-1. FEBS Lett. 1994. PMID: 8013631
-
Mechanical load-induced alterations in B-type natriuretic peptide gene expression.Can J Physiol Pharmacol. 2001 Aug;79(8):646-53. Can J Physiol Pharmacol. 2001. PMID: 11558673 Review.
-
Mechanisms of mechanical load-induced atrial natriuretic peptide secretion: role of endothelin, nitric oxide, and angiotensin II.J Mol Med (Berl). 1997 Nov-Dec;75(11-12):876-85. doi: 10.1007/s001090050179. J Mol Med (Berl). 1997. PMID: 9428620 Review.
Cited by
-
Timing and magnitude of systolic stretch affect myofilament activation and mechanical work.Am J Physiol Heart Circ Physiol. 2014 Aug 1;307(3):H353-60. doi: 10.1152/ajpheart.00233.2014. Epub 2014 May 30. Am J Physiol Heart Circ Physiol. 2014. PMID: 24878774 Free PMC article.
-
RNA-sequencing reveals altered skeletal muscle contraction, E3 ligases, autophagy, apoptosis, and chaperone expression in patients with critical illness myopathy.Skelet Muscle. 2019 Apr 16;9(1):9. doi: 10.1186/s13395-019-0194-1. Skelet Muscle. 2019. PMID: 30992050 Free PMC article.
-
Plasma N-Terminal Probrain Natriuretic Peptide, Vascular Endothelial Growth Factor, and Cardiac Troponin I as Novel Biomarkers of Hypertensive Disease and Target Organ Damage in Cats.J Vet Intern Med. 2017 May;31(3):650-660. doi: 10.1111/jvim.14655. Epub 2017 Apr 7. J Vet Intern Med. 2017. PMID: 28387019 Free PMC article.
-
Macro- and micromechanical remodelling in the fish atrium is associated with regulation of collagen 1 alpha 3 chain expression.Pflugers Arch. 2018 Aug;470(8):1205-1219. doi: 10.1007/s00424-018-2140-1. Epub 2018 Mar 28. Pflugers Arch. 2018. PMID: 29594338 Free PMC article.
-
Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy.Am J Physiol Regul Integr Comp Physiol. 2012 Oct 1;303(7):R689-99. doi: 10.1152/ajpregu.00548.2011. Epub 2012 Aug 8. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22874425 Free PMC article.
References
-
- Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE. Mechanisms underlying the increase in force and ca(2+) transient that follow stretch of cardiac muscle: A possible explanation of the anrep effect. Circ Res. 1999;85:716–722. - PubMed
-
- Barbosa ME, Alenina N, Bader M. Induction and analysis of cardiac hypertrophy in transgenic animal models. Methods Mol Med. 2005;112:339–352. - PubMed
-
- Beinlich CJ, Morgan HE. Control of growth in neonatal pig hearts. Mol Cell Biochem. 1993;119:3–9. - PubMed
-
- Bentivegna LA, Ablin LW, Kihara Y, Morgan JP. Altered calcium handling in left ventricular pressure-overload hypertrophy as detected with aequorin in the isolated, perfused ferret heart. Circ Res. 1991;69:1538–1545. - PubMed
-
- Berger HJ, et al. Continual electric field stimulation preserves contractile function of adult ventricular myocytes in primary culture. Am J Physiol. 1994;266:H341–H349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
