Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export
- PMID: 19673889
- PMCID: PMC11158120
- DOI: 10.1111/j.1349-7006.2009.01288.x
Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export
Abstract
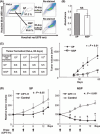
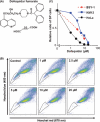
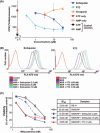
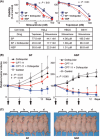
The ATP-binding cassette (ABC) transporters (ABC-T) actively efflux structurally and mechanistically unrelated anticancer drugs from cells. As a consequence, they can confer multidrug resistance (MDR) to cancer cells. ABC-T are also reported to be phenotypic markers and functional regulators of cancer stem/initiating cells (CSC) and believed to be associated with tumor initiation, progression, and relapse. Dofequidar fumarate, an orally active quinoline compound, has been reported to overcome MDR by inhibiting ABCB1/P-gp, ABCC1/MDR-associated protein 1, or both. Phase III clinical trials suggested that dofequidar had efficacy in patients who had not received prior therapy. Here we show that dofequidar inhibits the efflux of chemotherapeutic drugs and increases the sensitivity to anticancer drugs in CSC-like side population (SP) cells isolated from various cancer cell lines. Dofequidar treatment greatly reduced the cell number in the SP fraction. Estimation of ABC-T expression revealed that ABCG2/breast cancer resistance protein (BCRP) mRNA level, but not the ABCB1/P-gp or ABCC1/MDR-associated protein 1 mRNA level, in all the tested SP cells was higher than that in non-SP cells. The in vitro vesicle transporter assay clarified that dofequidar had the ability to suppress ABCG2/BCRP function. Dofequidar treatment sensitized SP cells to anticancer agents in vitro. We compared the antitumor efficacy of irinotecan (CPT-11) alone with that of CPT-11 plus dofequidar in xenografted SP cells. Although xenografted SP tumors showed resistance to CPT-11, treatment with CPT-11 plus dofequidar greatly reduced the SP-derived tumor growth in vivo. Our results suggest the possibility of selective eradication of CSC by inhibiting ABCG2/BCRP.
Figures


References
-
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev 2005; 5: 275–84. - PubMed
-
- Zhou S, Schuetz JD, Bunting KD et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nat Med 2001; 7: 1028–34. - PubMed
-
- Scharenberg CW, Harkey MA, Torok‐Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 2002; 99: 507–12. - PubMed
-
- Barile L, Messina E, Giacomello A, Marban E. Endogenous cardiac stem cells. Prog Cardiovasc Dis 2007; 50: 31–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

