SCORE Study Report 7: incidence of intravitreal silicone oil droplets associated with staked-on vs luer cone syringe design
- PMID: 19674727
- PMCID: PMC2780008
- DOI: 10.1016/j.ajo.2009.06.004
SCORE Study Report 7: incidence of intravitreal silicone oil droplets associated with staked-on vs luer cone syringe design
Abstract
Purpose: To evaluate the incidence of intravitreal silicone oil (SO) droplets associated with intravitreal injections using a staked-on vs luer cone syringe design in the SCORE (Standard Care vs COrticosteroid in REtinal Vein Occlusion) Study.
Design: Prospective, randomized, phase III clinical trial.
Methods: The incidence of intravitreal SO was compared among participants exposed to the staked-on syringe design, the luer cone syringe design, or both of the syringe designs in the SCORE Study, which evaluated intravitreal triamcinolone acetonide injection(s) for vision loss secondary to macular edema associated with central or branch retinal vein occlusion. Injections were given at baseline and 4-month intervals, based on treatment assignment and study-defined retreatment criteria. Because intravitreal SO was observed following injections in some participants, investigators were instructed, on September 22, 2006, to look for intravitreal SO at all study visits. On November 1, 2007, the luer cone syringe design replaced the staked-on syringe design.
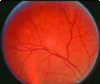
Results: A total of 464 participants received a total of 1,205 injections between November 4, 2004 and February 28, 2009. Intravitreal SO was noted in 141 of 319 participants (44%) exposed only to staked-on syringes, 11 of 87 (13%) exposed to both syringe designs, and 0 of 58 exposed only to luer cone syringes (P < .0001). Among participants with first injections after September 22, 2006, intravitreal SO was noted in 65 of 114 (57%) injected only with staked-on syringes compared with 0 of 58 injected only with luer cone syringes. Differential follow-up is unlikely to explain these results.
Conclusion: In the SCORE Study, luer cone syringe design is associated with a lower frequency of intravitreal SO droplet occurrence compared with the staked-on syringe design, likely attributable to increased residual space in the needle hub with the luer cone design.
Trial registration: ClinicalTrials.gov NCT00105027.
Figures



References
-
- Freund KB, Laud K, Eandi CM, Spaide RF. Silicone oil droplets following intravitreal injection. Retina. 2006;26:701–703. Medline. doi:10.1097/00006982-200607000-00021. - DOI - PubMed
-
- Bakri SJ, Ekdawi NS. Intravitreal silicone oil droplets after intravitreal drug injections. Retina. 2008;28:996–1001. Medline. doi:10.1097/IAE.0b013e31816c6868. - DOI - PubMed
-
- Manual of Policies and Procedures (MOPP) for the Standard Care vs. COrticosteroid for REtinal Vein Occlusion (SCORE) Study. Version 4.0. Bethesda, MD: National Eye Institute; NTIS order number PB2008-106870.
-
- Protocol for the Standard Care vs. COrticosteroid for REtinal Vein Occlusion (SCORE) Study. Version 7.0. Bethesda, MD: National Eye Institute; NTIS order number PB2008-113743.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

