Plakophilins: multifunctional scaffolds for adhesion and signaling
- PMID: 19674883
- PMCID: PMC3091506
- DOI: 10.1016/j.ceb.2009.07.002
Plakophilins: multifunctional scaffolds for adhesion and signaling
Abstract
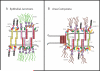
Armadillo family proteins known as plakophilins have been characterized as structural components of desmosomes that stabilize and strengthen adhesion by enhancing attachments with the intermediate filament cytoskeleton. However, plakophilins and their close relatives are emerging as versatile scaffolds for multiple signaling and metabolic processes that not only facilitate junction dynamics but also more globally regulate diverse cellular activities. While perturbation of plakophilin functions contribute to inherited diseases and cancer pathogenesis, the functional significance of the multiple PKP isoforms and the mechanisms by which their behaviors are regulated remain to be elucidated.
Figures

References
-
- Hatzfeld M, Green KJ, Sauter H. Targeting of p0071 to desmosomes and adherens junctions is mediated by different protein domains. J. Cell Sci. 2002;116:1219–1233. - PubMed
-
- Hofmann I, Schlechter T, Kuhn C, Hergt M, Franke WW. Protein p0071 - an armadillo plaque protein that characterizes a specific subtype of adherens junctions. J. Cell Sci. 2009;122:21–24. - PubMed
-
-
Hatzfeld M. Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesions? Biochim. Biophys. Acta. 2007;1773:69–77. A comprehensive review of the three main plakophilin isoforms outlines their genetic, structural, and functional similarities and differences in the context of cell adhesion, signaling and disease.
-
-
- Choi H-J, Weis WI. Structure of the armadillo repeat domain of plakophilin 1. J. Mol. Biol. 2005;346:367–376. - PubMed
-
- Heid HW, Schmidt A, Zimbelmann R, Schafer S, Winter-Simanowski S, Stumpp S, Keith M, Figge U, Schnolzer M, Franke WW. Cell type-specific desmosomal plaque proteins of the plakoglobin family: plakophilin 1 (band 6 protein) Differentiation. 1994;58:113–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

