Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials
- PMID: 19674936
- PMCID: PMC2748902
- DOI: 10.1016/S1470-2045(09)70201-3
Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials
Abstract
Background: Bisphosphonates might modulate the development of symptomatic bone metastases in men with prostate cancer. The Medical Research Council (MRC) PR05 and PR04 randomised controlled trials assessed the use of sodium clodronate, an oral, first-generation bisphosphonate. We report the final analyses of long-term survival data with additional follow-up in both trials.
Methods: 311 men with metastatic disease were recruited to PR05 between 1994 and 1998, and 508 men with non-metastatic disease were recruited to PR04 from 1994 to 1997. All men were treated according to the recruiting site's standard practice at the time: for metastatic disease, all men were starting or responding to long-term hormone therapy; for non-metastatic disease, most men had radiotherapy, hormone therapy, or both. Men were randomly assigned to take four tablets per day of sodium clodronate (2080 mg) or matching placebo for up to 3 years (metastatic disease) or 5 years (non-metastatic). Long-term overall survival was assessed on an intention-to-treat basis in all men at sites in England and Wales using data from the National Health Service Information Centre, which held data for 278 of 311 men in the PR05 trial and 471 of 508 men in the PR04 trial. These studies are registered International Standardised Randomised Controlled Trials, numbers ISRCTN38477744 (PR05) and ISRCTN61384873 (PR04).
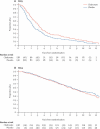
Findings: Of the 278 men with metastatic disease, 258 (93%) were reported to have died. Evidence of a benefit for those with metastatic disease from use of sodium clodronate compared with placebo was seen in overall survival (hazard ratio [HR] 0.77, 95% CI 0.60-0.98; p=0.032). Of the 471 men with non-metastatic disease, 281 (60%) were reported to have died, with no evidence of improvement in overall survival with clodronate compared with placebo (HR 1.12, 0.89-1.42; p=0.94).
Interpretation: Long-term data from these trials show that a first-generation bisphosphonate, sodium clodronate, improves overall survival in men with metastatic prostate cancer who are starting hormone therapy, but there is no evidence of an effect in men with non-metastatic prostate cancer.
Funding: UK MRC; and an education grant and free drug from Roche Products Ltd.
Figures

References
-
- Dearnaley D, Sydes M, Mason M. Oral sodium clodronate for metastatic prostate cancer—double-blind, placebo-controlled randomised trial: MRC PR05 (ISRCTN38477744) J Natl Cancer Inst. 2003;95:1300–1311. - PubMed
-
- Mason MD, Sydes MR, Glaholm J. Oral sodium clodronate for nonmetastatic prostate cancer—results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873) J Natl Cancer Inst. 2007;99:765–776. - PubMed
-
- Bolla M, Collette L, Blank L. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. - PubMed
-
- Widmark A, Klepp O, Solberg A. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2008;373:301–308. - PubMed
-
- D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

