A new subtype of frontotemporal lobar degeneration with FUS pathology
- PMID: 19674978
- PMCID: PMC2768659
- DOI: 10.1093/brain/awp214
A new subtype of frontotemporal lobar degeneration with FUS pathology
Abstract
Frontotemporal dementia (FTD) is a clinical syndrome with a heterogeneous molecular basis. The neuropathology associated with most FTD is characterized by abnormal cellular aggregates of either transactive response DNA-binding protein with Mr 43 kDa (TDP-43) or tau protein. However, we recently described a subgroup of FTD patients, representing around 10%, with an unusual clinical phenotype and pathology characterized by frontotemporal lobar degeneration with neuronal inclusions composed of an unidentified ubiquitinated protein (atypical FTLD-U; aFTLD-U). All cases were sporadic and had early-onset FTD with severe progressive behavioural and personality changes in the absence of aphasia or significant motor features. Mutations in the fused in sarcoma (FUS) gene have recently been identified as a cause of familial amyotrophic lateral sclerosis, with these cases reported to have abnormal cellular accumulations of FUS protein. Because of the recognized clinical, genetic and pathological overlap between FTD and amyotrophic lateral sclerosis, we investigated whether FUS might also be the pathological protein in aFTLD-U. In all our aFTLD-U cases (n = 15), FUS immunohistochemistry labelled all the neuronal inclusions and also demonstrated previously unrecognized glial pathology. Immunoblot analysis of protein extracted from post-mortem aFTLD-U brain tissue demonstrated increased levels of insoluble FUS. No mutations in the FUS gene were identified in any of our patients. These findings suggest that FUS is the pathological protein in a significant subgroup of sporadic FTD and reinforce the concept that FTD and amyotrophic lateral sclerosis are closely related conditions.
Figures

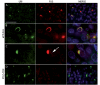

Comment in
-
With or without FUS, it is the anatomy that dictates the dementia phenotype.Brain. 2009 Nov;132(Pt 11):2906-8. doi: 10.1093/brain/awp286. Brain. 2009. PMID: 19861505 Free PMC article. No abstract available.
-
Very early-onset frontotemporal dementia with no family history predicts underlying fused in sarcoma pathology.Brain. 2010 Dec;133(Pt 12):e158; author reply e159. doi: 10.1093/brain/awq186. Epub 2010 Aug 7. Brain. 2010. PMID: 20693541 No abstract available.
References
-
- Aman P, Panagopoulos I, Lassen C, Fioretos T, Mencinger M, Toresson H, et al. Expression patterns of the human sarcoma-associated genes FUS and EWS and the genomic structure of FUS. Genomics. 1996;37:1–8. - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–11. - PubMed
-
- Baechtold H, Kuroda M, Sok J, Ron D, Lopez BS, Akhmedov AT. Human 75-kDa DNA-pairing protein is identical to the pro-oncoprotein TLD/FUS and is able to promote D-loop formation. J Biol Chem. 1999;274:34337–42. - PubMed
-
- Benajiba L, Le Ber I, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65:470–3. - PubMed

