Receptor tyrosine phosphatases control tracheal tube geometries through negative regulation of Egfr signaling
- PMID: 19675131
- PMCID: PMC2730367
- DOI: 10.1242/dev.033597
Receptor tyrosine phosphatases control tracheal tube geometries through negative regulation of Egfr signaling
Abstract
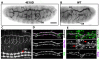
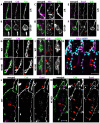
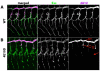
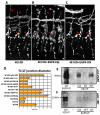
The formation of epithelial tubes with defined shapes and sizes is essential for organ development. We describe a unique tracheal tubulogenesis phenotype caused by loss of both Drosophila type III receptor tyrosine phosphatases (RPTPs), Ptp4E and Ptp10D. Ptp4E is the only widely expressed Drosophila RPTP, and is the last of the six fly RPTPs to be genetically characterized. We recently isolated mutations in Ptp4E, and discovered that, although Ptp4E null mutants have no detectable phenotypes, double mutants lacking both Ptp4E and Ptp10D display synthetic lethality at hatching owing to respiratory failure. In these double mutants, unicellular and terminal tracheal branches develop large bubble-like cysts that selectively incorporate apical cell surface markers. Cysts in unicellular branches are enlargements of the lumen that are sealed by adherens junctions, whereas cysts in terminal branches are cytoplasmic vacuoles. Cyst size and number are increased by tracheal expression of activated Egfr tyrosine kinase, and decreased by reducing Egfr levels. Ptp10D forms a complex with Egfr in transfected cells. Downregulation of Egfr signaling by the RPTPs is required for the construction of tubular lumens, whether extracellular or intracellular, by cells that undergo remodeling during branch morphogenesis. The Ptp4E Ptp10D phenotype represents the first evidence of an essential role for RPTPs in epithelial organ development. These findings might be relevant to organ development and disease in mammals, because PTPRJ (DEP-1), an ortholog of Ptp4E/Ptp10D, interacts with the hepatocyte growth factor receptor tyrosine kinase. PTPRJ corresponds to the murine Scc1 (suppressor of colon cancer) gene.
Figures



References
-
- Affolter, M. and Caussinus, E. (2008). Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development 135, 2055-2064. - PubMed
-
- Baumgartner, S., Littleton, J. T., Broadie, K., Bhat, M. A., Harbecke, R., Lengyel, J. A., Chiquet-Ehrismann, R., Prokop, A. and Bellen, H. J. (1996). A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 87, 1059-1068. - PubMed
-
- Beitel, G. J. and Krasnow, M. A. (2000). Genetic control of epithelial tube size in the Drosophila tracheal system. Development 127, 3271-3282. - PubMed
-
- Buff, E., Carmena, A., Gisselbrecht, S., Jimenez, F. and Michelson, A. M. (1998). Signalling by the Drosophila epidermal growth factor receptor is required for the specification and diversification of embryonic muscle progenitors. Development 125, 2075-2086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

