Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance
- PMID: 19675137
- PMCID: PMC2768161
- DOI: 10.2337/db09-0216
Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance
Abstract
Objective: We sought to determine whether exosome-like vesicles (ELVs) released from adipose tissue play a role in activation of macrophages and subsequent development of insulin resistance in a mouse model.
Research design and methods: ELVs released from adipose tissue were purified by sucrose gradient centrifugation and labeled with green fluorescent dye and then intravenously injected into B6 ob/ob mice (obese model) or B6 mice fed a high-fat diet. The effects of injected ELVs on the activation of macrophages were determined through analysis of activation markers by fluorescence-activated cell sorter and induction of inflammatory cytokines using an ELISA. Glucose tolerance and insulin tolerance were also evaluated. Similarly, B6 mice with different gene knockouts including TLR2, TLR4, MyD88, and Toll-interleukin-1 receptor (TIR) domain-containing adaptor protein inducing interferon-beta (TRIF) were also used for testing their responses to the injected ELVs.
Results: ELVs are taken up by peripheral blood monocytes, which then differentiate into activated macrophages with increased secretion of tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6). Injection of obELVs into wild-type C57BL/6 mice results in the development of insulin resistance. When the obELVs were intravenously injected into TLR4 knockout B6 mice, the levels of glucose intolerance and insulin resistance were much lower. RBP4 is enriched in the obELVs. Bone marrow-derived macrophages preincubated with recombinant RBP4 led to attenuation of obELV-mediated induction of IL-6 and TNF-alpha.
Conclusions: ELVs released by adipose tissue can act as a mode of communication between adipose tissues and macrophages. The obELV-mediated induction of TNF-alpha and IL-6 in macrophages and insulin resistance requires the TLR4/TRIF pathway.
Figures
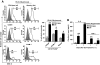

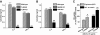

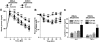
References
-
- Nakamachi T, Nomiyama T, Gizard F, et al. : PPARα agonists suppress osteopontin expression in macrophages and decrease plasma levels in patients with type 2 diabetes. Diabetes 2007;56:1662–1670 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

