Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis
- PMID: 19675149
- PMCID: PMC2754643
- DOI: 10.1104/pp.109.143701
Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis
Abstract
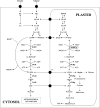
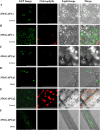
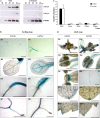
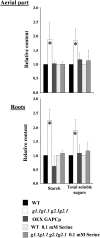
Glycolysis is a central metabolic pathway that, in plants, occurs in both the cytosol and the plastids. The glycolytic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) catalyzes the conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate with concomitant reduction of NAD(+) to NADH. Both cytosolic (GAPCs) and plastidial (GAPCps) GAPDH activities have been described. However, the in vivo functions of the plastidial isoforms remain unresolved. In this work, we have identified two Arabidopsis (Arabidopsis thaliana) chloroplast/plastid-localized GAPDH isoforms (GAPCp1 and GAPCp2). gapcp double mutants display a drastic phenotype of arrested root development, dwarfism, and sterility. In spite of their low gene expression level as compared with other GAPDHs, GAPCp down-regulation leads to altered gene expression and to drastic changes in the sugar and amino acid balance of the plant. We demonstrate that GAPCps are important for the synthesis of serine in roots. Serine supplementation to the growth medium rescues root developmental arrest and restores normal levels of carbohydrates and sugar biosynthetic activities in gapcp double mutants. We provide evidence that the phosphorylated pathway of Ser biosynthesis plays an important role in supplying serine to roots. Overall, these studies provide insights into the in vivo functions of the GAPCps in plants. Our results emphasize the importance of the plastidial glycolytic pathway, and specifically of GAPCps, in plant primary metabolism.
Figures






Comment in
-
A critical role of plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase in the control of plant metabolism and development.Plant Signal Behav. 2010 Jan;5(1):67-9. doi: 10.4161/psb.5.1.10200. Plant Signal Behav. 2010. PMID: 20592814 Free PMC article.
Similar articles
-
A critical role of plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase in the control of plant metabolism and development.Plant Signal Behav. 2010 Jan;5(1):67-9. doi: 10.4161/psb.5.1.10200. Plant Signal Behav. 2010. PMID: 20592814 Free PMC article.
-
Overexpression of the triose phosphate translocator (TPT) complements the abnormal metabolism and development of plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase mutants.Plant J. 2017 Mar;89(6):1146-1158. doi: 10.1111/tpj.13452. Epub 2017 Feb 11. Plant J. 2017. PMID: 27984670
-
Plastidial Glycolytic Glyceraldehyde-3-Phosphate Dehydrogenase Is an Important Determinant in the Carbon and Nitrogen Metabolism of Heterotrophic Cells in Arabidopsis.Plant Physiol. 2015 Nov;169(3):1619-37. doi: 10.1104/pp.15.00696. Epub 2015 Jul 1. Plant Physiol. 2015. PMID: 26134167 Free PMC article.
-
The SnRK1 Kinase as Central Mediator of Energy Signaling between Different Organelles.Plant Physiol. 2018 Feb;176(2):1085-1094. doi: 10.1104/pp.17.01404. Epub 2018 Jan 8. Plant Physiol. 2018. PMID: 29311271 Free PMC article. Review.
-
Physiology, phylogeny, early evolution, and GAPDH.Protoplasma. 2017 Sep;254(5):1823-1834. doi: 10.1007/s00709-017-1095-y. Epub 2017 Mar 6. Protoplasma. 2017. PMID: 28265765 Free PMC article. Review.
Cited by
-
Distinct plastid fructose bisphosphate aldolases function in photosynthetic and non-photosynthetic metabolism in Arabidopsis.J Exp Bot. 2021 May 4;72(10):3739-3755. doi: 10.1093/jxb/erab099. J Exp Bot. 2021. PMID: 33684221 Free PMC article.
-
Combining genetic diversity, informatics and metabolomics to facilitate annotation of plant gene function.Nat Protoc. 2010 Jun;5(6):1210-27. doi: 10.1038/nprot.2010.82. Epub 2010 Jun 10. Nat Protoc. 2010. PMID: 20539294
-
Genome-wide identification, characterization, interaction network and expression profile of GAPDH gene family in sweet orange (Citrus sinensis).PeerJ. 2019 Nov 14;7:e7934. doi: 10.7717/peerj.7934. eCollection 2019. PeerJ. 2019. PMID: 31741784 Free PMC article.
-
The Plastidial Glyceraldehyde-3-Phosphate Dehydrogenase Is Critical for Abiotic Stress Response in Wheat.Int J Mol Sci. 2019 Mar 4;20(5):1104. doi: 10.3390/ijms20051104. Int J Mol Sci. 2019. PMID: 30836662 Free PMC article.
-
Cytosolic phosphorylating glyceraldehyde-3-phosphate dehydrogenases affect Arabidopsis cellular metabolism and promote seed oil accumulation.Plant Cell. 2014 Jul;26(7):3023-35. doi: 10.1105/tpc.114.126946. Epub 2014 Jul 2. Plant Cell. 2014. PMID: 24989043 Free PMC article.
References
-
- Alaiz M, Navarro JL, Giron J, Vioque E (1992) Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate. J Chromatogr A 591: 181–186 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Backhausen JE, Vetter S, Baalmann E, Kitzmann C, Scheibe R (1998) NAD-dependent malate dehydrogenase and glyceraldehyde 3-phosphate dehydrogenase isoenzymes play an important role in dark metabolism of various plastid types. Planta 205: 359–366
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

