Processing and presentation of variant surface glycoprotein molecules to T cells in African trypanosomiasis
- PMID: 19675169
- PMCID: PMC2730433
- DOI: 10.4049/jimmunol.0802005
Processing and presentation of variant surface glycoprotein molecules to T cells in African trypanosomiasis
Abstract
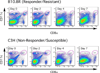
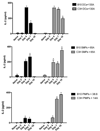
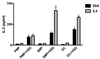
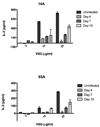
Th1 cell responses to the variant surface glycoprotein (VSG) of African trypanosomes play a critical role in controlling infection through the production of IFN-gamma, but the role of APCs in the induction and regulation of T cell-mediated protection is poorly understood. In this study, we have investigated the Ag presentation capabilities of dendritic cells (DCs) and macrophages during early trypanosome infection in relatively resistant responder and susceptible nonresponder mouse strains. Splenic DCs appeared to be the primary cell responsible for activating naive VSG-specific Th cell responses in resistant responder animals through the coordinated up-regulation of costimulatory molecules, secretion of IL-12, and presentation of VSG peptides to T cells in vivo. Splenic DC depletion and the down-regulation of costimulatory markers on splenic macrophages were observed in susceptible animals and may be associated with the inability of these animals to elicit a significant VSG-specific T cell response. In contrast to splenic APCs, peritoneal macrophages secreted NO, failed to activate naive Th cells in vitro, and presented relatively low levels of VSG peptides to T cells in vivo. Thus, VSG-specific Th1 cell responses may be determined by tissue- and cell-specific differences in Ag presentation. Additionally, all APCs from resistant and susceptible strains displayed a reduced ability to process and present newly encountered exogenous Ag, including new VSG molecules, during high parasitemia. Thus, initial uptake of VSG (or other trypanosome factors) may interfere with Ag presentation and have dramatic consequences for subsequent T cell responses to other proteins.
Figures






Similar articles
-
Resistance to the African trypanosomes is IFN-gamma dependent.J Immunol. 1998 Dec 15;161(12):6775-83. J Immunol. 1998. PMID: 9862708
-
Characterization of T helper cell responses to the trypanosome variant surface glycoprotein.J Immunol. 1993 Apr 1;150(7):2910-9. J Immunol. 1993. PMID: 8454863
-
Trypanosomes expressing a mosaic variant surface glycoprotein coat escape early detection by the immune system.Infect Immun. 2005 May;73(5):2690-7. doi: 10.1128/IAI.73.5.2690-2697.2005. Infect Immun. 2005. PMID: 15845470 Free PMC article.
-
Analysis of macrophage activation in African trypanosomiasis.J Leukoc Biol. 2001 May;69(5):685-90. J Leukoc Biol. 2001. PMID: 11358974 Review.
-
Induction and regulation of Trypanosoma brucei VSG-specific antibody responses.Parasitology. 2010 Dec;137(14):2041-9. doi: 10.1017/S003118200999165X. Epub 2009 Dec 22. Parasitology. 2010. PMID: 20025827 Review.
Cited by
-
Distinct Contributions of CD4+ and CD8+ T Cells to Pathogenesis of Trypanosoma brucei Infection in the Context of Gamma Interferon and Interleukin-10.Infect Immun. 2015 Jul;83(7):2785-95. doi: 10.1128/IAI.00357-15. Epub 2015 Apr 27. Infect Immun. 2015. PMID: 25916989 Free PMC article.
-
Oxidation by neutrophils-derived HOCl increases immunogenicity of proteins by converting them into ligands of several endocytic receptors involved in antigen uptake by dendritic cells and macrophages.PLoS One. 2015 Apr 7;10(4):e0123293. doi: 10.1371/journal.pone.0123293. eCollection 2015. PLoS One. 2015. PMID: 25849867 Free PMC article.
-
The Trypanosoma brucei gambiense secretome impairs lipopolysaccharide-induced maturation, cytokine production, and allostimulatory capacity of dendritic cells.Infect Immun. 2013 Sep;81(9):3300-8. doi: 10.1128/IAI.00125-13. Epub 2013 Jun 24. Infect Immun. 2013. PMID: 23798533 Free PMC article.
-
Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling.PLoS Pathog. 2010 Aug 12;6(8):e1001045. doi: 10.1371/journal.ppat.1001045. PLoS Pathog. 2010. PMID: 20714353 Free PMC article.
-
T-cell responses to the trypanosome variant surface glycoprotein are not limited to hypervariable subregions.Infect Immun. 2009 Jan;77(1):141-51. doi: 10.1128/IAI.00729-08. Epub 2008 Oct 20. Infect Immun. 2009. PMID: 18936180 Free PMC article.
References
-
- Cross GA. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975;71:393–417. - PubMed
-
- Dempsey WL, Mansfield JM. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983;130:405–411. - PubMed
-
- Levine RF, Mansfield JM. Genetics of resistance to the African trypanosomes. III. Variant-specific antibody responses of H-2-compatible resistant and susceptible mice. J Immunol. 1984;133:1564–1569. - PubMed
-
- Schleifer KW, Filutowicz H, Schopf LR, Mansfield JM. Characterization of T helper cell responses to the trypanosome variant surface glycoprotein. J Immunol. 1993;150:2910–2919. - PubMed
-
- Hertz CJ, Filutowicz H, Mansfield JM. Resistance to the African trypanosomes is IFN-gamma dependent. J Immunol. 1998;161:6775–6783. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

