Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate
- PMID: 19675183
- PMCID: PMC2781338
- DOI: 10.1152/ajprenal.00279.2009
Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate
Abstract
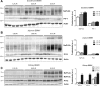
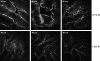
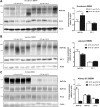
Hyperphosphatemia associated with chronic kidney disease is one of the factors that can promote vascular calcification, and intestinal P(i) absorption is one of the pharmacological targets that prevents it. The type II Na-P(i) cotransporter NaPi-2b is the major transporter that mediates P(i) reabsorption in the intestine. The potential role and regulation of other Na-P(i) transporters remain unknown. We have identified expression of the type III Na-P(i) cotransporter PiT-1 in the apical membrane of enterocytes. Na-P(i) transport activity and NaPi-2b and PiT-1 proteins are mostly expressed in the duodenum and jejunum of rat small intestine; their expression is negligible in the ileum. In response to a chronic low-P(i) diet, there is an adaptive response restricted to the jejunum, with increased brush border membrane (BBM) Na-P(i) transport activity and NaPi-2b, but not PiT-1, protein and mRNA abundance. However, in rats acutely switched from a low- to a high-P(i) diet, there is an increase in BBM Na-P(i) transport activity in the duodenum that is associated with an increase in BBM NaPi-2b protein abundance. Acute adaptive upregulation is restricted to the duodenum and induces an increase in serum P(i) that produces a transient postprandial hyperphosphatemia. Our study, therefore, indicates that Na-P(i) transport activity and NaPi-2b protein expression are differentially regulated in the duodenum vs. the jejunum and that postprandial upregulation of NaPi-2b could be a potential target for treatment of hyperphosphatemia.
Figures


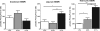
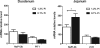
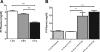


References
-
- Arima K, Hines ER, Kiela PR, Drees JB, Collins JF, Ghishan FK. Glucocorticoid regulation and glycosylation of mouse intestinal type IIb Na-Pi cotransporter during ontogeny. Am J Physiol Gastrointest Liver Physiol 283: G426–G434, 2002 - PubMed
-
- Bai L, Collins JF, Ghishan FK. Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am J Physiol Cell Physiol 279: C1135–C1143, 2000 - PubMed
-
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31: 607–617, 1998 - PubMed
-
- Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, St-Arnoud R, Murer H, Biber J. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1α-OHase-deficient mice. Am J Physiol Cell Physiol 288: C429–C434, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

