Highly efficient antiviral CD8+ T-cell induction by peptides coupled to the surfaces of liposomes
- PMID: 19675224
- PMCID: PMC2756846
- DOI: 10.1128/CVI.00116-09
Highly efficient antiviral CD8+ T-cell induction by peptides coupled to the surfaces of liposomes
Abstract
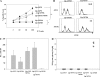
In previous studies, we have demonstrated that liposomes with differential lipid components display differential adjuvant effects when antigens (Ags) are chemically coupled to their surfaces. When ovalbumin was coupled to liposomes made by using unsaturated fatty acids, it was found to be presented not only to CD4(+) T cells but also to CD8(+) T cells and induced cytotoxic T lymphocytes (CTLs) which effectively eradicated the tumor from mice. In this study, we coupled liposomes to immunodominant CTL epitope peptides derived from lymphocytic choriomeningitis virus (LCMV) and evaluated its potency as an antiviral vaccine. The intramuscular immunization of mice with the peptide-liposome conjugates along with CpG resulted in the efficient induction of antiviral CD8(+) T-cell responses which conferred complete protection against not only LCMV Armstrong but also a highly virulent mutant strain, clone 13, that establishes persistent infections in immunocompetent mice. The intranasal vaccination induced mucosal immunity effective enough to protect mice from the virus challenge via the same route. Complete protection was achieved in mice even when the Ag dose was reduced to as low as 280 ng of liposomal peptide. This form of vaccination with a single CTL epitope induced Ag-specific memory CD8(+) T cells in the absence of CD4(+) T-cell help, which could be shown by the complete protection of CD4-knockout mice in 10 weeks as well as by the analysis of recall responses. Thus, surface-linked liposomal peptide might have a potential advantage for the induction of antiviral immunity.
Figures
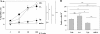





Similar articles
-
Coupling to the surface of liposomes alters the immunogenicity of hepatitis C virus-derived peptides and confers sterile immunity.Biochem Biophys Res Commun. 2013 Jan 4;430(1):183-9. doi: 10.1016/j.bbrc.2012.11.028. Epub 2012 Nov 15. Biochem Biophys Res Commun. 2013. PMID: 23159619 Free PMC article.
-
CpG-DNA activates in vivo T cell epitope presenting dendritic cells to trigger protective antiviral cytotoxic T cell responses.J Immunol. 2000 Mar 1;164(5):2372-8. doi: 10.4049/jimmunol.164.5.2372. J Immunol. 2000. PMID: 10679072
-
Archaeosomes induce long-term CD8+ cytotoxic T cell response to entrapped soluble protein by the exogenous cytosolic pathway, in the absence of CD4+ T cell help.J Immunol. 2000 Nov 1;165(9):5177-85. doi: 10.4049/jimmunol.165.9.5177. J Immunol. 2000. PMID: 11046050
-
CTLs Get SMAD When Pathogens Tell Them Where to Go.J Immunol. 2022 Sep 15;209(6):1025-1032. doi: 10.4049/jimmunol.2200345. J Immunol. 2022. PMID: 36130123 Free PMC article. Review.
-
Vaccine immunity against fungal infections.Curr Opin Immunol. 2014 Jun;28:27-33. doi: 10.1016/j.coi.2014.01.014. Epub 2014 Mar 3. Curr Opin Immunol. 2014. PMID: 24583636 Free PMC article. Review.
Cited by
-
Nanoscale Interaction Mechanisms of Antiviral Activity.ACS Pharmacol Transl Sci. 2023 Jan 10;6(2):220-228. doi: 10.1021/acsptsci.2c00195. eCollection 2023 Feb 10. ACS Pharmacol Transl Sci. 2023. PMID: 36798473 Free PMC article. Review.
-
Nanotechnology-facilitated vaccine development during the coronavirus disease 2019 (COVID-19) pandemic.Exploration (Beijing). 2022 Jul 21;2(5):20210082. doi: 10.1002/EXP.20210082. Online ahead of print. Exploration (Beijing). 2022. PMID: 35941992 Free PMC article. Review.
-
An overview on the field of micro- and nanotechnologies for synthetic Peptide-based vaccines.J Drug Deliv. 2011;2011:181646. doi: 10.1155/2011/181646. Epub 2011 Jun 15. J Drug Deliv. 2011. PMID: 21773041 Free PMC article.
-
Characterization of CD8+ T cell function and immunodominance generated with an H2O2-inactivated whole-virus vaccine.J Virol. 2012 Dec;86(24):13735-44. doi: 10.1128/JVI.02178-12. Epub 2012 Oct 10. J Virol. 2012. PMID: 23055558 Free PMC article.
-
Targeting cryptic epitope with modified antigen coupled to the surface of liposomes induces strong antitumor CD8 T-cell immune responses in vivo.Oncol Rep. 2015 Dec;34(6):2827-36. doi: 10.3892/or.2015.4299. Epub 2015 Sep 21. Oncol Rep. 2015. PMID: 26398429 Free PMC article.
References
-
- Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. - PMC - PubMed
-
- Bartholdy, C., A. Stryhn, J. P. Christensen, and A. R. Thomsen. 2004. Single-epitope DNA vaccination prevents exhaustion and facilitates a broad antiviral CD8+ T cell response during chronic viral infection. J. Immunol. 173:6284-6293. - PubMed
-
- Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297:2060-2063. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

