The potassium chloride cotransporter KCC-2 coordinates development of inhibitory neurotransmission and synapse structure in Caenorhabditis elegans
- PMID: 19675228
- PMCID: PMC2737711
- DOI: 10.1523/JNEUROSCI.1989-09.2009
The potassium chloride cotransporter KCC-2 coordinates development of inhibitory neurotransmission and synapse structure in Caenorhabditis elegans
Abstract
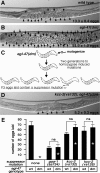
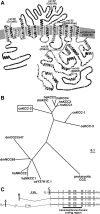
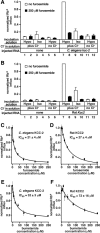
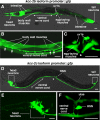
Chloride influx through GABA-gated chloride channels, the primary mechanism by which neural activity is inhibited in the adult mammalian brain, depends on chloride gradients established by the potassium chloride cotransporter KCC2. We used a genetic screen to identify genes important for inhibition of the hermaphrodite-specific motor neurons (HSNs) that stimulate Caenorhabditis elegans egg-laying behavior and discovered mutations in a potassium chloride cotransporter, kcc-2. Functional analysis indicates that, like mammalian KCCs, C. elegans KCC-2 transports chloride, is activated by hypotonic conditions, and is inhibited by the loop diuretic furosemide. KCC-2 appears to establish chloride gradients required for the inhibitory effects of GABA-gated and serotonin-gated chloride channels on C. elegans behavior. In the absence of KCC-2, chloride gradients appear to be altered in neurons and muscles such that normally inhibitory signals become excitatory. kcc-2 is transcriptionally upregulated in the HSN neurons during synapse development. Loss of KCC-2 produces a decrease in the synaptic vesicle population within mature HSN synapses, which apparently compensates for a lack of HSN inhibition, resulting in normal egg-laying behavior. Thus, KCC-2 coordinates the development of inhibitory neurotransmission with synapse maturation to produce mature neural circuits with appropriate activity levels.
Figures



Similar articles
-
Two types of chloride transporters are required for GABA(A) receptor-mediated inhibition in C. elegans.EMBO J. 2011 May 4;30(9):1852-63. doi: 10.1038/emboj.2011.83. Epub 2011 Mar 22. EMBO J. 2011. PMID: 21427702 Free PMC article.
-
A glial K(+) /Cl(-) cotransporter modifies temperature-evoked dynamics in Caenorhabditis elegans sensory neurons.Genes Brain Behav. 2016 Apr;15(4):429-40. doi: 10.1111/gbb.12260. Epub 2015 Nov 13. Genes Brain Behav. 2016. PMID: 26463820
-
An evolutionarily conserved switch in response to GABA affects development and behavior of the locomotor circuit of Caenorhabditis elegans.Genetics. 2015 Apr;199(4):1159-72. doi: 10.1534/genetics.114.173963. Epub 2015 Feb 2. Genetics. 2015. PMID: 25644702 Free PMC article.
-
Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases.Biochim Biophys Acta. 2010 Dec;1802(12):1150-8. doi: 10.1016/j.bbadis.2010.07.009. Epub 2010 Jul 15. Biochim Biophys Acta. 2010. PMID: 20637866 Free PMC article. Review.
-
The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures.Neurosurg Focus. 2008 Sep;25(3):E22. doi: 10.3171/FOC/2008/25/9/E22. Neurosurg Focus. 2008. PMID: 18759624 Review.
Cited by
-
Cell-type-specific promoters for C. elegans glia.J Neurogenet. 2020 Sep-Dec;34(3-4):335-346. doi: 10.1080/01677063.2020.1781851. Epub 2020 Jul 22. J Neurogenet. 2020. PMID: 32696701 Free PMC article.
-
Regulation of chromatin states and gene expression during HSN neuronal maturation is mediated by EOR-1/PLZF, MAU-2/cohesin loader, and SWI/SNF complex.Sci Rep. 2018 May 21;8(1):7942. doi: 10.1038/s41598-018-26149-2. Sci Rep. 2018. PMID: 29786685 Free PMC article.
-
Kinase-KCC2 coupling: Cl- rheostasis, disease susceptibility, therapeutic target.J Neurophysiol. 2016 Jan 1;115(1):8-18. doi: 10.1152/jn.00865.2015. Epub 2015 Oct 28. J Neurophysiol. 2016. PMID: 26510764 Free PMC article. Review.
-
Neuron cilia restrain glial KCC-3 to a microdomain to regulate multisensory processing.Cell Rep. 2024 Mar 26;43(3):113844. doi: 10.1016/j.celrep.2024.113844. Epub 2024 Feb 27. Cell Rep. 2024. PMID: 38421867 Free PMC article.
-
IRK-1 potassium channels mediate peptidergic inhibition of Caenorhabditis elegans serotonin neurons via a G(o) signaling pathway.J Neurosci. 2012 Nov 14;32(46):16285-95. doi: 10.1523/JNEUROSCI.2667-12.2012. J Neurosci. 2012. PMID: 23152612 Free PMC article.
References
-
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
