Overcoming macrophage-mediated axonal dieback following CNS injury
- PMID: 19675231
- PMCID: PMC2771342
- DOI: 10.1523/JNEUROSCI.1151-09.2009
Overcoming macrophage-mediated axonal dieback following CNS injury
Abstract
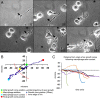
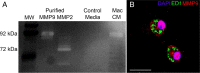
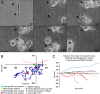
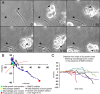
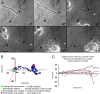
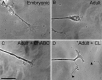
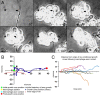
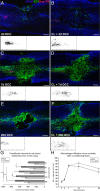
Trauma to the adult CNS initiates multiple processes including primary and secondary axotomy, inflammation, and glial scar formation that have devastating effects on neuronal regeneration. After spinal cord injury, the infiltration of phagocytic macrophages coincides with long-distance axonal retraction from the initial site of injury, a deleterious phenomenon known as axonal dieback. We have previously shown that activated macrophages directly induce long-distance retraction of dystrophic axons in an in vitro model of the glial scar. We hypothesized that treatments that are primarily thought to increase neuronal regeneration following spinal cord injury may in fact derive a portion of their beneficial effects from inhibition of macrophage-mediated axonal retraction. We analyzed the effects of protease inhibition, substrate modification, and neuronal preconditioning on macrophage-axon interactions using our established in vitro model. General inhibition of matrix metalloproteinases and specific inhibition of MMP-9 prevented macrophage-induced axonal retraction despite significant physical interactions between the two cell types, whereas inhibition of MMP-2 had no effect. Chondroitinase ABC-mediated digestion of the aggrecan substrate also prevented macrophage-induced axonal retraction in the presence of extensive macrophage-axon interactions. The use of a conditioning lesion to stimulate intrinsic neuronal growth potential in the absence of substrate modification likewise prevented macrophage-induced axonal retraction in vitro and in vivo following spinal cord injury. These data provide valuable insight into the cellular and molecular mechanisms underlying macrophage-mediated axonal retraction and demonstrate modifications that can alleviate the detrimental effects of this unfavorable phenomenon on the postlesion CNS.
Figures
References
-
- Baba T, Iwasaki S, Maruoka T, Suzuki A, Tomaru U, Ikeda H, Yoshiki T, Kasahara M, Ishizu A. Rat CD4+CD8+ macrophages kill tumor cells through an NKG2D- and granzyme/perforin-dependent mechanism. J Immunol. 2008;180:2999–3006. - PubMed
-
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. - PubMed
-
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. - PubMed
-
- Chandler S, Miller KM, Clements JM, Lury J, Corkill D, Anthony DC, Adams SE, Gearing AJ. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
