BC1 regulation of metabotropic glutamate receptor-mediated neuronal excitability
- PMID: 19675232
- PMCID: PMC2866649
- DOI: 10.1523/JNEUROSCI.3893-08.2009
BC1 regulation of metabotropic glutamate receptor-mediated neuronal excitability
Abstract
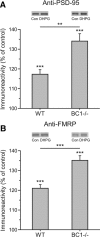
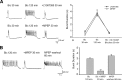
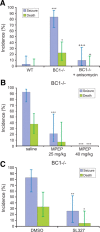
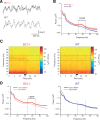
Regulatory RNAs have been suggested to contribute to the control of gene expression in eukaryotes. Brain cytoplasmic (BC) RNAs are regulatory RNAs that control translation initiation. We now report that neuronal BC1 RNA plays an instrumental role in the protein-synthesis-dependent implementation of neuronal excitation-repression equilibria. BC1 repression counter-regulates translational stimulation resulting from synaptic activation of group I metabotropic glutamate receptors (mGluRs). Absence of BC1 RNA precipitates plasticity dysregulation in the form of neuronal hyperexcitability, elicited by group I mGluR-stimulated translation and signaled through the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway. Dysregulation of group I mGluR function in the absence of BC1 RNA gives rise to abnormal brain function. Cortical EEG recordings from freely moving BC1(-/-) animals show that group I mGluR-mediated oscillations in the gamma frequency range are significantly elevated. When subjected to sensory stimulation, these animals display an acute group I mGluR-dependent propensity for convulsive seizures. Inadequate RNA control in neurons is thus causally linked to heightened group I mGluR-stimulated translation, neuronal hyperexcitability, heightened gamma band oscillations, and epileptogenesis. These data highlight the significance of small RNA control in neuronal plasticity.
Figures


References
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
-
- Barciszewski J, Erdmann VA, editors. Georgetown, TX: Landes Bioscience; 2003. Noncoding RNAs: molecular biology and molecular medicine.
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
-
- Brosius J. Waste not, want not: transcript excess in multicellular eukaryotes. Trends Genet. 2005;21:287–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
