Endothelin-1 regulates oligodendrocyte development
- PMID: 19675238
- PMCID: PMC2754292
- DOI: 10.1523/JNEUROSCI.0822-09.2009
Endothelin-1 regulates oligodendrocyte development
Abstract
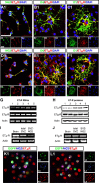
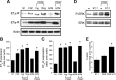
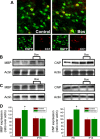
In the postnatal brain, oligodendrocyte progenitor cells (OPCs) arise from the subventricular zone (SVZ) and migrate into the developing white matter, where they differentiate into oligodendrocytes and myelinate axons. The mechanisms regulating OPC migration and differentiation are not fully defined. The present study demonstrates that endothelin-1 (ET-1) is an astrocyte-derived signal that regulates OPC migration and differentiation. OPCs in vivo and in culture express functional ET(A) and ET(B) receptors, which mediate ET-1-induced ERK (extracellular signal-regulated kinase) and CREB (cAMP response element-binding protein) phosphorylation. ET-1 exerts both chemotactic and chemokinetic effects on OPCs to enhance cell migration; it also prevents lineage progression from the O4(+) to the O1(+) stage without affecting cell proliferation. Astrocyte-conditioned medium stimulates OPC migration in culture through ET receptor activation, whereas multiphoton time-lapse imaging shows that selective ET receptor antagonists or anti-ET-1 antibodies inhibit OPC migration from the SVZ. Inhibition of ET receptor activity also derepresses OPC differentiation in the corpus callosum in slice cultures. Our findings indicate that ET-1 is a soluble astrocyte-derived signal that regulates OPC migration and differentiation during development.
Figures






Similar articles
-
NMDA receptor couples Rac1-GEF Tiam1 to direct oligodendrocyte precursor cell migration.Glia. 2013 Dec;61(12):2078-99. doi: 10.1002/glia.22578. Epub 2013 Oct 7. Glia. 2013. PMID: 24123220
-
Myelin Proteolipid Protein Complexes with αv Integrin and AMPA Receptors In Vivo and Regulates AMPA-Dependent Oligodendrocyte Progenitor Cell Migration through the Modulation of Cell-Surface GluR2 Expression.J Neurosci. 2015 Aug 26;35(34):12018-32. doi: 10.1523/JNEUROSCI.5151-14.2015. J Neurosci. 2015. PMID: 26311781 Free PMC article.
-
Stimulation of endothelin B receptors in astrocytes induces cAMP response element-binding protein phosphorylation and c-fos expression via multiple mitogen-activated protein kinase signaling pathways.J Neurosci. 2001 Nov 15;21(22):8842-53. doi: 10.1523/JNEUROSCI.21-22-08842.2001. J Neurosci. 2001. PMID: 11698596 Free PMC article.
-
Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone.Glia. 2012 Oct;60(10):1451-67. doi: 10.1002/glia.22365. Epub 2012 Jun 17. Glia. 2012. PMID: 22706957 Free PMC article.
-
The endocannabinoid 2-arachidonoylglycerol regulates oligodendrocyte progenitor cell migration.Biochem Pharmacol. 2018 Nov;157:180-188. doi: 10.1016/j.bcp.2018.09.006. Epub 2018 Sep 6. Biochem Pharmacol. 2018. PMID: 30195734
Cited by
-
Hepatoma Derived Growth Factor Enhances Oligodendrocyte Genesis from Subventricular Zone Precursor Cells.ASN Neuro. 2022 Jan-Dec;14:17590914221086340. doi: 10.1177/17590914221086340. ASN Neuro. 2022. PMID: 35293825 Free PMC article.
-
Astrocyte-derived endothelin-1 inhibits remyelination through notch activation.Neuron. 2014 Feb 5;81(3):588-602. doi: 10.1016/j.neuron.2013.11.015. Neuron. 2014. PMID: 24507193 Free PMC article.
-
Newly Identified Deficiencies in the Multiple Sclerosis Central Nervous System and Their Impact on the Remyelination Failure.Biomedicines. 2022 Mar 30;10(4):815. doi: 10.3390/biomedicines10040815. Biomedicines. 2022. PMID: 35453565 Free PMC article. Review.
-
Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury.J Physiol. 2016 Jul 1;594(13):3539-52. doi: 10.1113/JP270895. Epub 2016 Mar 29. J Physiol. 2016. PMID: 26857216 Free PMC article. Review.
-
Oligodendrocyte regeneration: Its significance in myelin replacement and neuroprotection in multiple sclerosis.Neuropharmacology. 2016 Nov;110(Pt B):633-643. doi: 10.1016/j.neuropharm.2015.10.010. Epub 2015 Oct 22. Neuropharmacology. 2016. PMID: 26474658 Free PMC article. Review.
References
-
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. - PubMed
-
- Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
