Age-dependent impairment of spine morphology and synaptic plasticity in hippocampal CA1 neurons of a presenilin 1 transgenic mouse model of Alzheimer's disease
- PMID: 19675248
- PMCID: PMC6664983
- DOI: 10.1523/JNEUROSCI.1856-09.2009
Age-dependent impairment of spine morphology and synaptic plasticity in hippocampal CA1 neurons of a presenilin 1 transgenic mouse model of Alzheimer's disease
Abstract
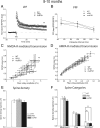
Presenilin 1 (PS1) mutations are responsible for a majority of early onset familial Alzheimer's disease (FAD) cases, in part by increasing the production of Abeta peptides. However, emerging evidence suggests other possible effects of PS1 on synaptic dysfunction where PS1 might contribute to the pathology independent of Abeta. We chose to study the L286V mutation, an aggressive FAD mutation which has never been analyzed at the electrophysiological and morphological levels. In addition, we analyzed for the first time the long term effects of wild-type human PS1 overexpression. We investigated the consequences of the overexpression of either wild-type human PS1 (hPS1) or the L286V mutated PS1 variant (mutPS1) on synaptic functions by analyzing synaptic plasticity and associated spine density changes from 3 to 15 months of age. We found that mutPS1 induces a transient increase observed only in 4- to 5-month-old mutPS1 animals in NMDA receptor (NMDA-R)-mediated responses and LTP compared with hPS1 mice and nontransgenic littermates. The increase in synaptic functions is concomitant with an increase in spine density. With increasing age, however, we found that the overexpression of human wild-type PS1 progressively decreased NMDA-R-mediated synaptic transmission and LTP, without neurodegeneration. These results identify for the first time a transient increase in synaptic function associated with L286V mutated PS1 variant in an age-dependent manner. In addition, they support the view that the PS1 overexpression promotes synaptic dysfunction in an Abeta-independent manner and underline the crucial role of PS1 during both normal and pathological aging.
Figures




References
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. - PubMed
-
- Barrow PA, Empson RM, Gladwell SJ, Anderson CM, Killick R, Yu X, Jefferys JG, Duff K. Functional phenotype in transgenic mice expressing mutant human presenilin-1. Neurobiol Dis. 2000;7:119–126. - PubMed
-
- Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50:77–82. - PubMed
-
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
