Activity-dependent modulation of glutamatergic signaling in the developing rat dorsal horn by early tissue injury
- PMID: 19675290
- PMCID: PMC2775379
- DOI: 10.1152/jn.00520.2009
Activity-dependent modulation of glutamatergic signaling in the developing rat dorsal horn by early tissue injury
Abstract
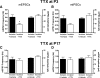
Tissue injury in early life can produce distinctive effects on pain processing, but little is known about the underlying neural mechanisms. Neonatal inflammation modulates excitatory synapses in spinal nociceptive circuits, but it is unclear whether this results directly from altered afferent input. Here we investigate excitatory and inhibitory synaptic transmission in the rat superficial dorsal horn following neonatal hindlimb surgical incision using in vitro patch-clamp recordings and test the effect of blocking peripheral nerve activity on the injury-evoked changes. Surgical incision through the skin and muscle of the hindlimb at postnatal day 3 (P3) or P10 selectively increased the frequency, but not amplitude, of glutamatergic miniature excitatory postsynaptic currents (mEPSCs) recorded 2-3 days after injury, without altering miniature inhibitory postsynaptic current frequency or amplitude at this time point. Meanwhile, incision at P17 failed to affect excitatory or inhibitory synaptic function at 2-3 days postinjury. The elevated mEPSC frequency was accompanied by increased inward rectification of evoked alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated currents, but no change in AMPAR/N-methyl-D-aspartate receptor ratios, and was followed by a persistent reduction in mEPSC frequency by 9-10 days postinjury. Prolonged blockade of primary afferent input from the time of injury was achieved by administration of bupivacaine hydroxide or tetrodotoxin to the sciatic nerve at P3. The increase in mEPSC frequency evoked by P3 incision was prevented by blocking sciatic nerve activity. These results demonstrate that increased afferent input associated with peripheral tissue injury selectively modulates excitatory synaptic drive onto developing spinal sensory neurons and that the enhanced glutamatergic signaling in the dorsal horn following neonatal surgical incision is activity dependent.
Figures



References
-
- Baba H, Doubell TP, Moore KA, Woolf CJ. Silent NMDA receptor-mediated synapses are developmentally regulated in the dorsal horn of the rat spinal cord. J Neurophysiol 83: 955–962, 2000 - PubMed
-
- Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J Neurophysiol 96: 579–590, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

