Secretory state regulates Zn2+ transport in gastric parietal cell of the rabbit
- PMID: 19675302
- PMCID: PMC2770737
- DOI: 10.1152/ajpcell.00577.2008
Secretory state regulates Zn2+ transport in gastric parietal cell of the rabbit
Abstract
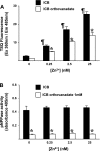
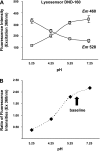
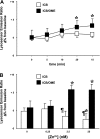
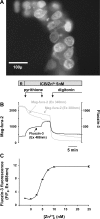
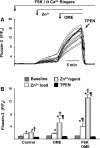
Secretory compartments of neurons, endocrine cells, and exocrine glands are acidic and contain high levels of labile Zn2+. Previously, we reported evidence that acidity is regulated, in part, by the content of Zn2+ in the secretory [i.e., tubulovesicle (TV)] compartment of the acid-secreting gastric parietal cell. Here we report studies focusing on the mechanisms of Zn2+ transport by the TV compartment in the mammalian (rabbit) gastric parietal cell. Uptake of Zn2+ by isolated TV structures was monitored with a novel application of the fluorescent Zn2+ reporter N-(6-methoxy-8-quinolyl)-para-toluenesulfonamide (TSQ). Uptake was suppressed by removal of external ATP or blockade of H+-K+-ATPase that mediates luminal acid secretion. Uptake was diminished with dissipation of the proton gradient across the TV membrane, suggesting Zn2+/H+ antiport as the connection between Zn2+ uptake and acidity in the TV lumen. In isolated gastric glands loaded with the reporter fluozin-3, inhibition of H+-K+-ATPase arrested the flow of Zn(2+) from the cytoplasm to the TV compartment and secretory stimulation with forskolin enhanced vectorial movement of cytoplasmic Zn2+ into the tubulovesicle/lumen (TV/L) compartment. Our findings suggest that Zn2+ accumulation in the TV/L compartment is physiologically coupled to secretion of acid. These findings offer novel insight into mechanisms regulating Zn2+ homeostasis in the gastric parietal cell and potentially other cells in which acidic subcellular compartments serve signature functional roles.
Figures




Similar articles
-
Divalent cations regulate acidity within the lumen and tubulovesicle compartment of gastric parietal cells.Gastroenterology. 2004 Jan;126(1):182-95. doi: 10.1053/j.gastro.2003.10.068. Gastroenterology. 2004. PMID: 14699499
-
Functional association between K+-Cl- cotransporter-4 and H+,K+-ATPase in the apical canalicular membrane of gastric parietal cells.J Biol Chem. 2009 Jan 2;284(1):619-629. doi: 10.1074/jbc.M806562200. Epub 2008 Nov 4. J Biol Chem. 2009. PMID: 18984587
-
Ion transport studies with H+-K+-ATPase-rich vesicles: implications for HCl secretion and parietal cell physiology.Am J Physiol. 1985 Jun;248(6 Pt 1):G595-607. doi: 10.1152/ajpgi.1985.248.6.G595. Am J Physiol. 1985. PMID: 2408481 Review.
-
Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation.Am J Physiol Gastrointest Liver Physiol. 2008 Jul;295(1):G99-G111. doi: 10.1152/ajpgi.00389.2007. Epub 2008 May 15. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18483183 Free PMC article.
-
Apical recycling of the gastric parietal cell H,K-ATPase.Annu Rev Physiol. 2010;72:273-96. doi: 10.1146/annurev-physiol-021909-135744. Annu Rev Physiol. 2010. PMID: 20148676 Review.
Cited by
-
Gastric and colonic zinc transporter ZIP11 (Slc39a11) in mice responds to dietary zinc and exhibits nuclear localization.J Nutr. 2013 Dec;143(12):1882-8. doi: 10.3945/jn.113.184457. Epub 2013 Oct 2. J Nutr. 2013. PMID: 24089422 Free PMC article.
-
Bitter Taste Receptor TAS2R43 Co-Regulates Mechanisms of Gastric Acid Secretion and Zinc Homeostasis.Int J Mol Sci. 2025 Jun 23;26(13):6017. doi: 10.3390/ijms26136017. Int J Mol Sci. 2025. PMID: 40649796 Free PMC article.
-
Demand for Zn2+ in acid-secreting gastric mucosa and its requirement for intracellular Ca2+.PLoS One. 2011;6(6):e19638. doi: 10.1371/journal.pone.0019638. Epub 2011 Jun 15. PLoS One. 2011. PMID: 21698273 Free PMC article.
-
Monochloramine-induced toxicity and dysregulation of intracellular Zn2+ in parietal cells of rabbit gastric glands.Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G170-8. doi: 10.1152/ajpgi.00355.2009. Epub 2010 Apr 29. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20430873 Free PMC article.
-
Antioxidant pre-treatment prevents omeprazole-induced toxicity in an in vitro model of infectious gastritis.Free Radic Biol Med. 2010 Sep 1;49(5):786-91. doi: 10.1016/j.freeradbiomed.2010.05.034. Epub 2010 Jun 8. Free Radic Biol Med. 2010. PMID: 20554018 Free PMC article.
References
-
- Agnew BJ, Duman JG, Watson CL, Coling DE, Forte JG. Cytological transformations associated with parietal cell stimulation: critical steps in the activation cascade. J Cell Sci 112: 2639–2646, 1999 - PubMed
-
- Akagi K, Nagao T, Urushidani T. Reconstitution of acid secretion in digitonin-permeabilized rabbit gastric glands. Identification of cytosolic regulatory factors. J Biol Chem 276: 28171–28172, 2001 - PubMed
-
- Ammar DA, Zhou R, Forte JG, Yao X. Syntaxin 3 is required for cAMP-induced acid secretion: streptolysin O-permeabilized gastric gland model. Am J Physiol Gastrointest Liver Physiol 282: G23–G33, 2002 - PubMed
-
- Andrews JC, Nolan JP, Hammerstedt RH, Bavister BD. Characterization of N-(6-methoxy-8-quinolyl)-p-toluenesulfonamide for the detection of zinc in living sperm cells. Cytometry 21: 153–159, 1995 - PubMed
-
- Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem 260: 2719–2727, 1985 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

