Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes
- PMID: 19675303
- PMCID: PMC2777402
- DOI: 10.1152/ajpcell.00283.2009
Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes
Abstract
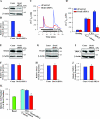
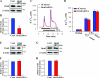
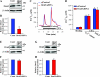
Ca(2+) entry through store-operated channels (SOCs) in the plasma membrane plays an important role in regulation of vascular smooth muscle contraction, tone, and cell proliferation. The C-type transient receptor potential (TRPC) channels have been proposed as major candidates for SOCs in vascular smooth muscle. Recently, two families of transmembrane proteins, Orai [also known as Ca(2+) release-activated Ca(2+) channel modulator (CRACM)] and stromal interacting molecule 1 (STIM1), were shown to be essential for the activation of SOCs mainly in nonexcitable cells. Here, using small interfering RNA, we show that Orai1 plays an essential role in activating store-operated Ca(2+) entry (SOCE) in primary cultured proliferating human aortic smooth muscle cells (hASMCs), whereas Orai2 and Orai3 do not contribute to SOCE. Knockdown of Orai1 protein expression significantly attenuated SOCE. Moreover, inhibition of Orai1 downregulated expression of Na(+)/Ca(2+) exchanger type 1 (NCX1) and plasma membrane Ca(2+) pump isoform 1 (PMCA1). The rate of cytosolic free Ca(2+) concentration decay after Ca(2+) transients in Ca(2+)-free medium was also greatly decreased under these conditions. This reduction of Ca(2+) extrusion, presumably via NCX1 and PMCA1, may be a compensation for the reduced SOCE. Immunocytochemical observations indicate that Orai1 and NCX1 are clustered in plasma membrane microdomains. Cell proliferation was attenuated in hASMCs with disrupted Orai1 expression and reduced SOCE. Thus Orai1 appears to be a critical component of SOCE in proliferating vascular smooth muscle cells, and may therefore be a key player during vascular growth and remodeling.
Figures



References
-
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium 33: 345–356, 2003 - PubMed
-
- Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium 42: 213–223, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

