Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis
- PMID: 19675306
- PMCID: PMC2911571
- DOI: 10.1165/rcmb.2009-0118OC
Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis
Abstract
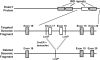
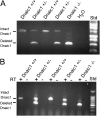
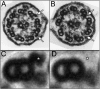
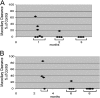
Studies of primary ciliary dyskinesia (PCD) have been hampered by the lack of a suitable animal model because disruption of essential ciliary genes in mice results in a high incidence of lethal hydrocephalus. To develop a viable mouse model for long-term studies of PCD, we have generated a transgenic mouse line in which two conserved exons of the mouse intermediate dynein chain gene, Dnaic1, are flanked by loxP sites (Dnaic1(flox/flox)). Dnaic1 is the murine homolog of human DNAI1, which is mutated in approximately 10% of human PCD cases. These mice have been crossed with mice expressing a tamoxifen-inducible Cre recombinase (CreER). Treatment of adult Dnaic1(flox/flox)/CreER(+/-) mice with tamoxifen results in an almost complete deletion of Dnaic1 with no evidence of hydrocephalus. Treated animals have reduced levels of full-length Dnaic1 mRNA, and electron micrographs of cilia demonstrate a loss of outer dynein arm structures. In treated Dnaic1(flox/flox)/CreER(+/-) animals, mucociliary clearance (MCC) was reduced over time. After approximately 3 months, no MCC was observed in the nasopharynx, whereas in the trachea, MCC was observed for up to 6 months, likely reflecting a difference in the turnover of ciliated cells in these tissues. All treated animals developed severe rhinosinusitis, demonstrating the importance of MCC to the health of the upper airways. However, no evidence of lung disease was observed up to 11 months after Dnaic1 deletion, suggesting that other mechanisms are able to compensate for the lack of MCC in the lower airways of mice. This model will be useful for the study of the pathogenesis and treatment of PCD.
Figures



Similar articles
-
Restoring ciliary function to differentiated primary ciliary dyskinesia cells with a lentiviral vector.Gene Ther. 2014 Mar;21(3):253-61. doi: 10.1038/gt.2013.79. Epub 2014 Jan 23. Gene Ther. 2014. PMID: 24451115 Free PMC article.
-
Mice with a Deletion of Rsph1 Exhibit a Low Level of Mucociliary Clearance and Develop a Primary Ciliary Dyskinesia Phenotype.Am J Respir Cell Mol Biol. 2019 Sep;61(3):312-321. doi: 10.1165/rcmb.2017-0387OC. Am J Respir Cell Mol Biol. 2019. PMID: 30896965 Free PMC article.
-
Inhaled DNAI1 mRNA therapy for treatment of primary ciliary dyskinesia.Proc Natl Acad Sci U S A. 2025 May 6;122(18):e2421915122. doi: 10.1073/pnas.2421915122. Epub 2025 Apr 28. Proc Natl Acad Sci U S A. 2025. PMID: 40294271 Free PMC article.
-
Genetic causes of bronchiectasis: primary ciliary dyskinesia.Respiration. 2007;74(3):252-63. doi: 10.1159/000101783. Respiration. 2007. PMID: 17534128 Review.
-
Ciliary Dyneins and Dynein Related Ciliopathies.Cells. 2021 Jul 25;10(8):1885. doi: 10.3390/cells10081885. Cells. 2021. PMID: 34440654 Free PMC article. Review.
Cited by
-
Comparative genome-wide survey of single nucleotide variation uncovers the genetic diversity and potential biomedical applications among six Macaca species.Int J Mol Sci. 2018 Oct 11;19(10):3123. doi: 10.3390/ijms19103123. Int J Mol Sci. 2018. PMID: 30314376 Free PMC article.
-
Sox2 modulates Lef-1 expression during airway submucosal gland development.Am J Physiol Lung Cell Mol Physiol. 2014 Apr 1;306(7):L645-60. doi: 10.1152/ajplung.00157.2013. Epub 2014 Jan 31. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24487391 Free PMC article.
-
CRISPR/Cas9-Mediated Rapid Generation of Multiple Mouse Lines Identified Ccdc63 as Essential for Spermiogenesis.Int J Mol Sci. 2015 Oct 16;16(10):24732-50. doi: 10.3390/ijms161024732. Int J Mol Sci. 2015. PMID: 26501274 Free PMC article.
-
Deletion of airway cilia results in noninflammatory bronchiectasis and hyperreactive airways.Am J Physiol Lung Cell Mol Physiol. 2014 Jan;306(2):L162-9. doi: 10.1152/ajplung.00095.2013. Epub 2013 Nov 8. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24213915 Free PMC article.
-
Continuous mucociliary transport by primary human airway epithelial cells in vitro.Am J Physiol Lung Cell Mol Physiol. 2015 Jul 15;309(2):L99-108. doi: 10.1152/ajplung.00024.2015. Epub 2015 May 15. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25979076 Free PMC article.
References
-
- Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med 1996;154:1868–1902. - PubMed
-
- Leigh MW. Primary ciliary dyskinesia. In: Chernick V, Boat TF, editors. Disorders of the respiratory tract of children. 6 ed. Philadelphia, PA: W.B. Saunders; 1998. pp. 819–825.
-
- Kennedy MP, Ostrowski LE. Primary ciliary dyskinesia and upper airway diseases. Curr Allergy Asthma Rep 2006;6:513–517. - PubMed
-
- Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 2004;169:459–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

