Identification of a putative Crf splice variant and generation of recombinant antibodies for the specific detection of Aspergillus fumigatus
- PMID: 19675673
- PMCID: PMC2721682
- DOI: 10.1371/journal.pone.0006625
Identification of a putative Crf splice variant and generation of recombinant antibodies for the specific detection of Aspergillus fumigatus
Abstract
Background: Aspergillus fumigatus is a common airborne fungal pathogen for humans. It frequently causes an invasive aspergillosis (IA) in immunocompromised patients with poor prognosis. Potent antifungal drugs are very expensive and cause serious adverse effects. Their correct application requires an early and specific diagnosis of IA, which is still not properly achievable. This work aims to a specific detection of A. fumigatus by immunofluorescence and the generation of recombinant antibodies for the detection of A. fumigatus by ELISA.
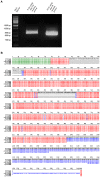
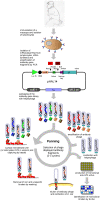
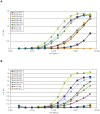
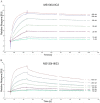
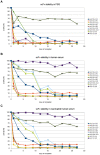
Results: The A. fumigatus antigen Crf2 was isolated from a human patient with proven IA. It is a novel variant of a group of surface proteins (Crf1, Asp f9, Asp f16) which belong to the glycosylhydrolase family. Single chain fragment variables (scFvs) were obtained by phage display from a human naive antibody gene library and an immune antibody gene library generated from a macaque immunized with recombinant Crf2. Two different selection strategies were performed and shown to influence the selection of scFvs recognizing the Crf2 antigen in its native conformation. Using these antibodies, Crf2 was localized in growing hyphae of A. fumigatus but not in spores. In addition, the antibodies allowed differentiation between A. fumigatus and related Aspergillus species or Candida albicans by immunofluorescence microscopy. The scFv antibody clones were further characterized for their affinity, the nature of their epitope, their serum stability and their detection limit of Crf2 in human serum.
Conclusion: Crf2 and the corresponding recombinant antibodies offer a novel approach for the early diagnostics of IA caused by A. fumigatus.
Conflict of interest statement
Figures





References
-
- Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–22. - PubMed
-
- Marr KA, Patterson T, Denning D. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect Dis Clin North Am. 2002;16:875–94. - PubMed
-
- Latgé JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–9. - PubMed
-
- Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, et al. Utility of galactomannan enzyme immunoassay and (1,3) beta-D-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol. 2009;47:129–33. doi: 10.1128/JCM.00506-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

