Acute lung affection in an endurance-trained man under amiodarone medication
- PMID: 19675720
- PMCID: PMC2703251
Acute lung affection in an endurance-trained man under amiodarone medication
Abstract
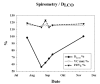
Patients undergoing treatment with amiodarone can develop severe pulmonary side effects. This effect, which is often highly underestimated, can lead to dyspnea, pneumonitis, and further fibrosis. A recent change in the labeling of amdiodarone by the American Food and Drug Administration (FDA) supports this suspicion. Tracing the symptoms back to the causing agent can be difficult, as shown in our report. The subject of this case report is an endurance-trained 65 year old male marathon runner who appeared with atrial fibrillation during a routine check up in autumn 2003. After medical cardioversion with flecainide a complaint free interval of 8 months was followed by a relapse, which resulted in a change of medication to amiodarone. Due to misunderstandings the patient kept on taking the amiodarone loading dose for six weeks and returned with severe dyspnea on exertion. Losses in CO diffusing capacity, a lowered macrophages count and a positive lymphocyte transformation test were the only first hand clinical evidence of amiodarone intoxication, despite the sensation of dyspnea. This case shows that special care has to be taken in treatment with amiodarone. Side effects can be hard to trace and do not evidently show a clear connection to amiodarone.
Wir beschreiben den Fall eines 65-jährigen Ausdauersportlers, der sich im Herbst 2003 mit Vorhofflimmern vorstellte. Nach medikamentöser Kardioversion mit Flecainid folgte ein beschwerdefreies Intervall von acht Monaten. Rezidive des Vorhofflimmerns erforderten die Umstellung auf Amiodaron. Aufgrund von Missverständnissen nahm der Patient die Aufsättigungsdosis über einen Zeitraum von sechs Wochen ein, wobei die ersten vier Wochen beschwerdefrei verliefen und sich über die nächsten zwei Wochen eine ausgeprägte Atemnot entwickelte. Dieser Fallbericht zeigt, dass die Behandlung mit Amiodaron besonderer Sorgfalt bedarf. Nebenwirkungen sind bisweilen sehr schwer erkennbar und zeigen nicht immer eine klare Beziehung zu der Amiodaroneinnahme.
Keywords: amiodarone; dyspnoe; pneumonitis; vital capacity.
Figures
References
-
- Gleadhill IC, Wise RA, Schonfeld SA, Scott PP, Guarnieri T, Levine JH, Griffith LS, Veltri EP. Serial lung function testing in patients treated with amiodarone: a prospective study. Am J Med. 1989;86(1):4–10. - PubMed
-
- Reasor MJ, Kacew S. An evaluation of possible mechanisms underlying amiodarone-induced pulmonary toxicity. Proc Soc Exp Biol Med. 1996;212(4):297–304. - PubMed
-
- Ashrafian H, Davey P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest. 2001;120(1):275–282. - PubMed
-
- Burns KE, Piliotis E, Garcia BM, Ferguson KA. Amiodarone pulmonary, neuromuscular and ophthalmological toxicity. Can Respir J. 2000;7(2):193–197. - PubMed
-
- Forth W, Henschler D, Rummel W, Förstermann U, Starke K, editors. Allgemeine und spezielle Pharmakologie und Toxikologie. 8. Aufl. München: Urban und Fischer; 2001.
LinkOut - more resources
Full Text Sources