Improvement of wound healing by water-filtered infrared-A (wIRA) in patients with chronic venous stasis ulcers of the lower legs including evaluation using infrared thermography
- PMID: 19675738
- PMCID: PMC2703263
Improvement of wound healing by water-filtered infrared-A (wIRA) in patients with chronic venous stasis ulcers of the lower legs including evaluation using infrared thermography
Abstract
Background: Water-filtered infrared-A (wIRA) is a special form of heat radiation with a high tissue-penetration and with a low thermal burden to the surface of the skin. wIRA is able to improve essential and energetically meaningful factors of wound healing by thermal and non-thermal effects.
Aim of the study: prospective study (primarily planned randomised, controlled, blinded, de facto with one exception only one cohort possible) using wIRA in the treatment of patients with recalcitrant chronic venous stasis ulcers of the lower legs with thermographic follow-up.
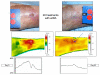
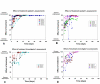
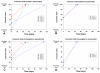
Methods: 10 patients (5 males, 5 females, median age 62 years) with 11 recalcitrant chronic venous stasis ulcers of the lower legs were treated with water-filtered infrared-A and visible light irradiation (wIRA(+VIS), Hydrosun radiator type 501, 10 mm water cuvette, water-filtered spectrum 550-1400 nm) or visible light irradiation (VIS; only possible in one patient). The uncovered wounds of the patients were irradiated two to five times per week for 30 minutes at a standard distance of 25 cm (approximately 140 mW/cm(2) wIRA and approximately 45 mW/cm(2) VIS). Treatment continued for a period of up to 2 months (typically until closure or nearly closure of the ulcer). The main variable of interest was "percent change of ulcer size over time" including complete wound closure. Additional variables of interest were thermographic image analysis, patient's feeling of pain in the wound, amount of pain medication, assessment of the effect of the irradiation (by patient and by clinical investigator), assessment of feeling of the wound area (by patient), assessment of wound healing (by clinical investigator) and assessment of the cosmetic state (by patient and by clinical investigator). For these assessments visual analogue scales (VAS) were used.
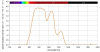
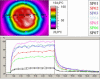
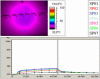
Results: The study showed a complete or nearly complete healing of lower leg ulcers in 7 patients and a clear reduction of ulcer size in another 2 of 10 patients, a clear reduction of pain and pain medication consumption (e.g. from 15 to 0 pain tablets per day), and a normalization of the thermographic image (before the beginning of the therapy typically hyperthermic rim of the ulcer with relative hypothermic ulcer base, up to 4.5 degrees C temperature difference). In one patient the therapy of an ulcer of one leg was performed with the fully active radiator (wIRA(+VIS)), while the therapy of an ulcer of the other leg was made with a control group radiator (only VIS without wIRA), showing a clear difference in favour of the wIRA treatment. All mentioned VAS ratings improved remarkably during the period of irradiation treatment, representing an increased quality of life. Failures of complete or nearly complete wound healing were seen only in patients with arterial insufficiency, in smokers or in patients who did not have venous compression garment therapy.
Discussion and conclusions: wIRA can alleviate pain considerably (with an impressive decrease of the consumption of analgesics) and accelerate wound healing or improve a stagnating wound healing process and diminish an elevated wound exudation and inflammation both in acute and in chronic wounds (in this study shown in chronic venous stasis ulcers of the lower legs) and in problem wounds including infected wounds. In chronic recalcitrant wounds complete healing is achieved, which was not reached before. Other studies have shown that even without a disturbance of wound healing an acute wound healing process can be improved (e.g. reduced pain) by wIRA. wIRA is a contact-free, easily used and pleasantly felt procedure without consumption of material with a good penetration effect, which is similar to solar heat radiation on the surface of the earth in moderate climatic zones. Wound healing and infection defence (e.g. granulocyte function including antibacterial oxygen radical formation of the granulocytes) are critically dependent on a sufficient energy supply (and on sufficient oxygen). The good clinical effect of wIRA on wounds and also on problem wounds and wound infections can be explained by the improvement of both the energy supply and the oxygen supply (e.g. for the granulocyte function). wIRA causes as a thermal effect in the tissue an improvement in three decisive factors: tissue oxygen partial pressure, tissue temperature and tissue blood flow. Besides this non-thermal effects of infrared-A by direct stimulation of cells and cellular structures with reactions of the cells have also been described. It is concluded that wIRA can be used to improve wound healing, to reduce pain, exudation, and inflammation and to increase quality of life.
Hintergrund: Wassergefiltertes Infrarot A (wIRA) ist eine spezielle Form der Wärmestrahlung mit hoher Gewebepenetration bei geringer thermischer Oberflächenbelastung. wIRA vermag über thermische und nicht-thermische Effekte wesentliche und energetisch bedeutsame Faktoren der Wundheilung zu verbessern.
Ziel der Studie: prospektive Studie (primär randomisiert, kontrolliert, verblindet geplant, de facto mit einer Ausnahme nur eine Kohorte möglich) mit wassergefiltertem Infrarot A (wIRA) in der Therapie von Patienten mit therapierefraktären chronischen venösen Unterschenkel-Ulzera mit thermographischer Verlaufskontrolle.
Methoden: 10 Patienten (5 Männer, 5 Frauen, Median des Alters 62 Jahre) mit 11 therapierefraktären chronischen venösen Unterschenkel-Ulzera wurden mit wassergefiltertem Infrarot A und sichtbarem Licht (wIRA(+VIS), Hydrosun®-Strahler Typ 501, 10 mm Wasserküvette, wassergefiltertes Spektrum 550–1400 nm) oder mit sichtbarem Licht (VIS; nur bei einem Patienten möglich) bestrahlt. Die unbedeckten Wunden der Patienten wurden zwei- bis fünfmal pro Woche über bis zu 2 Monate (typischerweise bis zum Wundschluss oder Fast-Wundschluss des Ulkus) für jeweils 30 Minuten mit einem Standardabstand von 25 cm bestrahlt (ungefähr 140 mW/cm2 wIRA und ungefähr 45 mW/cm2 VIS). Hauptzielvariable war die „prozentuale Änderung der Ulkusgröße über die Zeit“ einschließlich des kompletten Wundschlusses. Zusätzliche Zielvariablen waren thermographische Bildanalyse, Schmerzempfinden des Patienten in der Wunde, Schmerzmittelverbrauch, Einschätzung des Effekts der Bestrahlung (durch Patient und durch klinischen Untersucher), Einschätzung des Patienten des Gefühls im Wundbereich, Einschätzung der Wundheilung durch den klinischen Untersucher sowie Einschätzung des kosmetischen Zustandes (durch Patienten und durch klinischen Untersucher). Für diese Erhebungen wurden visuelle Analogskalen (VAS) verwendet.
Ergebnisse: Die Studie ergab eine vollständige oder fast vollständige Abheilung der Unterschenkel-Ulzera bei 7 Patienten sowie eine deutliche Ulkusverkleinerung bei 2 weiteren der 10 Patienten, eine bemerkenswerte Minderung der Schmerzen und des Schmerzmittelverbrauchs (von z.B. 15 auf 0 Schmerztabletten täglich) und eine Normalisierung des thermographischen Bildes (vor Therapiebeginn typischerweise hyperthermer Ulkusrandwall mit relativ hypothermem Ulkusgrund, bis zu 4,5°C Temperaturdifferenz). Bei einem Patienten wurde ein Ulkus an einem Bein mit dem Vollwirkstrahler (wIRA(+VIS)) therapiert, während ein Ulkus am anderen Bein mit einem Kontrollgruppenstrahler (nur VIS, ohne wIRA) behandelt wurde, was einen deutlichen Unterschied zugunsten der wIRA-Therapie zeigte. Alle aufgeführten VAS-Einschätzungen verbesserten sich während der Bestrahlungstherapie-Periode sehr stark, was einer verbesserten Lebensqualität entsprach. Ein kompletter oder fast kompletter Wundschluss wurde nur bei Patienten mit peripherer arterieller Verschlusskrankheit, Rauchern oder Patienten mit fehlender venöser Kompressionstherapie nicht erreicht.
Diskussion und Schlussfolgerungen: wIRA kann sowohl bei akuten Wunden als auch bei chronischen Wunden (in dieser Studie für chronische venöse Unterschenkelulzera gezeigt) und Problemwunden einschließlich infizierter Wunden Schmerzen deutlich mindern (mit eindrucksvoller Abnahme des Schmerzmittelverbrauchs) und die Wundheilung beschleunigen oder einen stagnierenden Wundheilungsprozess verbessern sowie eine erhöhte Wundsekretion und Entzündung mindern.
Bei chronischen therapierefraktären Wunden werden vollständige Abheilungen erreicht, die zuvor nicht erreicht wurden. Andere Studien haben sogar ohne Wundheilungsstörung eine Verbesserung (z.B. Schmerzreduktion) der akuten Wundheilung durch wIRA gezeigt.
wIRA ist ein kontaktfreies, verbrauchsmaterialfreies, leicht anzuwendendes, als angenehm empfundenes Verfahren mit guter Tiefenwirkung, das der Sonnenwärmestrahlung auf der Erdoberfläche in gemäßigten Klimazonen nachempfunden ist.
Wundheilung und Infektionsabwehr (z.B. Granulozytenfunktion einschließlich antibakterieller Sauerstoffradikalbildung der Granulozyten) hängen ganz entscheidend von einer ausreichenden Energieversorgung (und von ausreichend Sauerstoff) ab. Die gute klinische Wirkung von wIRA auf Wunden und auch auf Problemwunden und Wundinfektionen lässt sich über die Verbesserung sowohl der Energiebereitstellung als auch der Sauerstoffversorgung (z.B. für die Granulozytenfunktion) erklären. wIRA bewirkt als thermischen Effekt im Gewebe eine Verbesserung von drei entscheidenden Faktoren: Sauerstoffpartialdruck im Gewebe, Gewebetemperatur und Gewebedurchblutung. Daneben wurden auch nicht-thermische Effekte von Infrarot A durch direkte Reizsetzung auf Zellen und zelluläre Strukturen mit Reaktionen der Zellen beschrieben.
Es wird geschlossen, dass wIRA verwendet werden kann, um Wundheilung zu verbessern, Schmerzen, Sekretion und Entzündung zu reduzieren und die Lebensqualität zu steigern.
Keywords: chronic venous stasis ulcers of the lower legs; energy supply; infrared thermography; oxygen supply; problem wounds; prospective study; quality of life; reduction of pain; thermographic image analysis; tissue blood flow; tissue oxygen partial pressure; tissue temperature; visual analogue scales (VAS); water-filtered infrared-A (wIRA); wound healing; wound infections.
Figures


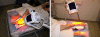



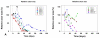




References
-
- Sarkar PK, Ballantyne S. Management of leg ulcers. Postgrad Med J. 2000;76:674–682. DOI: 10.1136/pmj.76.901.674. Available from: http://dx.doi.org/10.1136/pmj.76.901.674. - DOI - PMC - PubMed
-
- Jaschke E, Zabernigg A, Gattringer C. Recombinant human granulocyte-macrophage colony-stimulating factor applied locally in low doses enhances healing and prevents recurrence of chronic venous ulcers. Int J Dermatol. 1999;38:380–386. - PubMed
-
- Skin Substitute Consensus Development Panel. Nonoperative management of venous leg ulcers: Evolving role of skin substitutes. Vasc Surg. 1999;33:197–210. DOI: 10.1177/153857449903300217. Available from: http://dx.doi.org/10.1177/153857449903300217. - DOI
-
- Krieg T, Ferguson M. Key session: Growth factors: From bench to bedside I. Z Wundheilung - J Wound Healing. 2005;special issue 2:64.
-
- Tallman P, Muscare E, Carson P, Eaglstein WH, Falanga V. Initial rate of healing predicts complete healing of venous ulcers. Arch Dermatol. 1997;133:1231–1234. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
