A (13)C{(31)P} REDOR NMR investigation of the role of glutamic acid residues in statherin- hydroxyapatite recognition
- PMID: 19678690
- PMCID: PMC3246581
- DOI: 10.1021/la901647n
A (13)C{(31)P} REDOR NMR investigation of the role of glutamic acid residues in statherin- hydroxyapatite recognition
Abstract
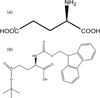
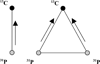
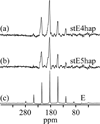
The side chain carboxyl groups of acidic proteins found in the extra-cellular matrix (ECM) of mineralized tissues play a key role in promoting or inhibiting the growth of minerals such as hydroxyapatite (HAP), the principal mineral component of bone and teeth. Among the acidic proteins found in the saliva is statherin, a 43-residue tyrosine-rich peptide that is a potent lubricant in the salivary pellicle and an inhibitor of both HAP crystal nucleation and growth. Three acidic amino acids-D1, E4, and E5-are located in the N-terminal 15 amino acid segment, with a fourth amino acid, E26, located outside the N-terminus. We have utilized (13)C{(31)P} REDOR NMR to analyze the role played by acidic amino acids in the binding mechanism of statherin to the HAP surface by measuring the distance between the delta-carboxyl (13)C spins of the three glutamic acid side chains of statherin (residues E4, E5, E26) and (31)P spins of the phosphate groups at the HAP surface. (13)C{(31)P} REDOR studies of glutamic-5-(13)C acid incorporated at positions E4 and E26 indicate a (13)C-(31)P distance of more than 6.5 A between the side chain carboxyl (13)C spin of E4 and the closest (31)P in the HAP surface. In contrast, the carboxyl (13)C spin at E5 has a much shorter (13)C-(31)P internuclear distance of 4.25 +/- 0.09 A, indicating that the carboxyl group of this side chain interacts directly with the surface. (13)C T(1rho) and slow-spinning MAS studies indicate that the motions of the side chains of E4 and E5 are more restricted than that of E26. Together, these results provide further insight into the molecular interactions of statherin with HAP surfaces.
Figures





Similar articles
-
Homonuclear and heteronuclear NMR studies of a statherin fragment bound to hydroxyapatite crystals.J Phys Chem B. 2006 May 11;110(18):9324-32. doi: 10.1021/jp056644g. J Phys Chem B. 2006. PMID: 16671751
-
A REDOR NMR study of a phosphorylated statherin fragment bound to hydroxyapatite crystals.J Am Chem Soc. 2005 Jul 6;127(26):9350-1. doi: 10.1021/ja050910m. J Am Chem Soc. 2005. PMID: 15984845
-
Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization.J Biol Chem. 1992 Mar 25;267(9):5968-76. J Biol Chem. 1992. PMID: 1313424
-
Molecular recognition at the protein-hydroxyapatite interface.Crit Rev Oral Biol Med. 2003;14(5):370-6. doi: 10.1177/154411130301400507. Crit Rev Oral Biol Med. 2003. PMID: 14530305 Review.
-
The structure, dynamics, and energetics of protein adsorption-lessons learned from adsorption of statherin to hydroxyapatite.Magn Reson Chem. 2007 Dec;45 Suppl 1:S32-47. doi: 10.1002/mrc.2123. Magn Reson Chem. 2007. PMID: 18172904 Review.
Cited by
-
Ubiquitin immobilized on mesoporous MCM41 silica surfaces - Analysis by solid-state NMR with biophysical and surface characterization.Biointerphases. 2017 May 31;12(2):02D414. doi: 10.1116/1.4983273. Biointerphases. 2017. PMID: 28565916 Free PMC article.
-
The Role of Basic Amino Acids in the Molecular Recognition of Hydroxyapatite by Statherin using Solid State NMR.Surf Sci. 2010 Aug 15;604(15-16):L39-L42. doi: 10.1016/j.susc.2010.02.026. Surf Sci. 2010. PMID: 20676391 Free PMC article.
-
Toward a structure determination method for biomineral-associated protein using combined solid- state NMR and computational structure prediction.Structure. 2010 Dec 8;18(12):1678-87. doi: 10.1016/j.str.2010.09.013. Structure. 2010. PMID: 21134646 Free PMC article.
-
Strongly bound citrate stabilizes the apatite nanocrystals in bone.Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22425-9. doi: 10.1073/pnas.1009219107. Epub 2010 Dec 2. Proc Natl Acad Sci U S A. 2010. PMID: 21127269 Free PMC article.
-
Solid state NMR investigation of intact human bone quality: balancing issues and insight into the structure at the organic-mineral interface.J Phys Chem C Nanomater Interfaces. 2012 Mar 15;116(10):6320-6331. doi: 10.1021/jp2125312. Epub 2012 Feb 21. J Phys Chem C Nanomater Interfaces. 2012. PMID: 22822414 Free PMC article.
References
-
- Schoen FJ, Levy RJ. Ann. Thorac. Surg. 2005;79:1072–1080. - PubMed
-
- Coe FL, Parks JH, Asplin JR. N. Engl. J. Med. 1992;327:1141–1152. - PubMed
-
- McCarty DJ. DM-Dis. Mon. 1994;40:259–299. - PubMed
-
- Giachelli CM. J. Am. Soc. Nephrol. 2004;15:2959–2564. - PubMed
-
- Margolis HC, Beniash E, Fowler CE. J. Dent. Res. 2006;85:775–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

