Nosiheptide biosynthesis featuring a unique indole side ring formation on the characteristic thiopeptide framework
- PMID: 19678698
- PMCID: PMC2763056
- DOI: 10.1021/cb900133x
Nosiheptide biosynthesis featuring a unique indole side ring formation on the characteristic thiopeptide framework
Abstract
Nosiheptide (NOS), belonging to the e series of thiopeptide antibiotics that exhibit potent activity against various bacterial pathogens, bears a unique indole side ring system and regiospecific hydroxyl groups on the characteristic macrocyclic core. Here, cloning, sequencing, and characterization of the nos gene cluster from Streptomyces actuosus ATCC 25421 as a model for this series of thiopeptides has unveiled new insights into their biosynthesis. Bioinformatics-based sequence analysis and in vivo investigation into the gene functions show that NOS biosynthesis shares a common strategy with recently characterized b or c series thiopeptides for forming the characteristic macrocyclic core, which features a ribosomally synthesized precursor peptide with conserved posttranslational modifications. However, it apparently proceeds via a different route for tailoring the thiopeptide framework, allowing the final product to exhibit the distinct structural characteristics of e series thiopeptides, such as the indole side ring system. Chemical complementation supports the notion that the S-adenosylmethionine-dependent protein NosL may play a central role in converting tryptophan to the key 3-methylindole moiety by an unusual carbon side chain rearrangement, most likely via a radical-initiated mechanism. Characterization of the indole side ring-opened analogue of NOS from the nosN mutant strain is consistent with the proposed methyltransferase activity of its encoded protein, shedding light into the timing of the individual steps for indole side ring biosynthesis. These results also suggest the feasibility of engineering novel thiopeptides for drug discovery by manipulating the NOS biosynthetic machinery.
Figures



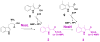



References
-
- Bagley MC, Dale JW, Merritt EA, Xiong X. Thiopeptide antibiotics. Chem Rev. 2005;105:685–714. - PubMed
-
- Leet JE, Li W, Ax HA, Matson JA, Huang S, Huang R, Cantone JL, Drexler D, Dalterio RA, Lam KS. Nocathiacins, new thiazolyl peptide antibiotics from Nocardia sp. II. Isolation, characterization, and structure determination. J Antibiot (Tokyo) 2003;56:232–242. - PubMed
-
- Li W, Leet JE, Ax HA, Gustavson DR, Brown DM, Turner L, Brown K, Clark J, Yang H, Fung-Tomc J, Lam KS. Nocathiacins, new thiazolyl peptide antibiotics from Nocardia sp. I. Taxonomy, fermentation and biological activities. J Antibiot (Tokyo) 2003;56:226–231. - PubMed
-
- Naidu BN, Sorenson ME, Zhang Y, Kim OK, Matiskella JD, Wichtowski JA, Connolly TP, Li W, Lam KS, Bronson JJ, Pucci MJ, Clark JM, Ueda Y. Nocathiacin I analogues: synthesis, in vitro and in vivo biological activity of novel semi-synthetic thiazolyl peptide antibiotics. Bioorg Med Chem Lett. 2004;14:5573–5577. - PubMed
-
- Li W, Huang S, Liu X, Leet JE, Cantone JL, Lam KS. N-Demethylation of nocathiacin I via photo-oxidation. Bioorg Med Chem Lett. 2008;18:4051–4053. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

