Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture
- PMID: 19678762
- PMCID: PMC2804768
- DOI: 10.1089/ten.TEA.2009.0164
Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture
Abstract
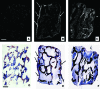
We report engineering of half-centimeter-sized bone constructs created in vitro using human adipose-derived stem cells (hASCs), decellularized bone scaffolds, and perfusion bioreactors. The hASCs are easily accessible, can be used in an autologous fashion, are rapidly expanded in culture, and are capable of osteogenic differentiation. hASCs from four donors were characterized for their osteogenic capacity, and one representative cell population was used for tissue engineering experiments. Culture-expanded hASCs were seeded on fully decellularized native bone scaffolds (4 mm diameter x 4 mm thick), providing the necessary structural and mechanical environment for osteogenic differentiation, and cultured in bioreactors with medium perfusion. The interstitial flow velocity was set to a level necessary to maintain cell viability and function throughout the construct volume (400 microm/s), via enhanced mass transport. After 5 weeks of cultivation, the addition of osteogenic supplements (dexamethasone, sodium-beta-glycerophosphate, and ascorbic acid-2-phosphate) to culture medium significantly increased the construct cellularity and the amounts of bone matrix components (collagen, bone sialoprotein, and bone osteopontin). Medium perfusion markedly improved the distribution of cells and bone matrix in engineered constructs. In summary, a combination of hASCs, decellularized bone scaffold, perfusion culture, and osteogenic supplements resulted in the formation of compact and viable bone tissue constructs.
Figures




References
-
- Salgado A.J. Coutinho O.P. Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743. - PubMed
-
- Giannoudis P.V. Dinopoulos H. Tsiridis E. Bone substitutes: an update. Injury. 2005;36:20. - PubMed
-
- Lecanda F. Avioli L.V. Cheng S.L. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J Cell Biochem. 1997;67:386. - PubMed
-
- Bruder S.P. Jaiswal N. Haynesworth S.E. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278. - PubMed
-
- Meinel L. Karageorgiou V. Fajardo R. Snyder B. Shinde-Patil V. Zichner L. Kaplan D. Langer R. Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

