Obesity-blocking neurons in Drosophila
- PMID: 19679073
- PMCID: PMC2742587
- DOI: 10.1016/j.neuron.2009.07.021
Obesity-blocking neurons in Drosophila
Erratum in
- Neuron. 2009 Oct 29;64(2):290-1
Abstract
In mammals, fat store levels are communicated by leptin and insulin signaling to brain centers that regulate food intake and metabolism. By using transgenic manipulation of neural activity, we report the isolation of two distinct neuronal populations in flies that perform a similar function, the c673a-Gal4 and fruitless-Gal4 neurons. When either of these neuronal groups is silenced, fat store levels increase. This change is mediated through an increase in food intake and altered metabolism in c673a-Gal4-silenced flies, while silencing fruitless-Gal4 neurons alters only metabolism. Hyperactivation of either neuronal group causes depletion of fat stores by increasing metabolic rate and decreasing fatty acid synthesis. Altering the activities of these neurons causes changes in expression of genes known to regulate fat utilization. Our results show that the fly brain measures fat store levels and can induce changes in food intake and metabolism to maintain them within normal limits.
Figures
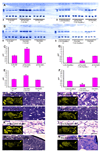
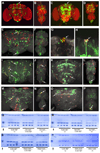
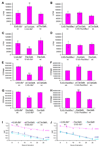


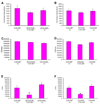
Comment in
-
Too fat to fly? New brain circuits regulate obesity in Drosophila.Neuron. 2009 Aug 13;63(3):279-81. doi: 10.1016/j.neuron.2009.07.023. Neuron. 2009. PMID: 19679067
References
-
- Armstrong JD, Kaiser K. The Study of Drosophila Brain Development. In: Houdebine LM, editor. Transgenic animals - generation and use. Harwood Academic Publishers; 1997.
-
- Barcelo H, Stewart MJ. Altering Drosophila S6 kinase activity is consistent with a role for S6 kinase in growth. Genesis. 34:83–85. - PubMed
-
- Barsh G, Schwartz M. Genetic approaches to studying energy balance: perception and integration. Nature Reviews. 2002;3(8):589–600. - PubMed
-
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. - PubMed
-
- Billeter J, Goodwin SF. Characterization of Drosophila fruitless-gal4 transgenes reveals expression in male-specific fruitless neurons and innervation of male reproductive structures. J. Comp. Neurol. 2004;475:270–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

