Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations
- PMID: 19679077
- PMCID: PMC2758537
- DOI: 10.1016/j.neuron.2009.06.020
Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations
Abstract
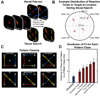
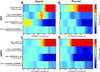
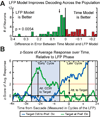
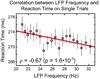
Attention regulates the flood of sensory information into a manageable stream, and so understanding how attention is controlled is central to understanding cognition. Competing theories suggest visual search involves serial and/or parallel allocation of attention, but there is little direct, neural evidence for either mechanism. Two monkeys were trained to covertly search an array for a target stimulus under visual search (endogenous) and pop-out (exogenous) conditions. Here, we present neural evidence in the frontal eye fields (FEF) for serial, covert shifts of attention during search but not pop-out. Furthermore, attention shifts reflected in FEF spiking activity were correlated with 18-34 Hz oscillations in the local field potential, suggesting a "clocking" signal. This provides direct neural evidence that primates can spontaneously adopt a serial search strategy and that these serial covert shifts of attention are directed by the FEF. It also suggests that neuron population oscillations may regulate the timing of cognitive processing.
Figures

References
-
- Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. - PubMed
-
- Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. - PubMed
-
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci. 1999;2:549–554. - PubMed
-
- Bisley JW, Goldberg ME. Neuronal Activity in the Lateral Intraparietal Area and Spatial Attention. Science. 2003;299:81–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

