Anti-inflammatory treatment in AD mice protects against neuronal pathology
- PMID: 19679126
- PMCID: PMC2864355
- DOI: 10.1016/j.expneurol.2009.07.032
Anti-inflammatory treatment in AD mice protects against neuronal pathology
Abstract
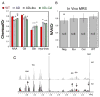
Prior studies suggest that non-steroidal anti-inflammatory drugs (NSAIDs) may lower the incidence of Alzheimer's disease (AD) and delay onset or slow progression of symptoms in mouse models of AD. We examined the effects of chronic NSAID treatment in order to determine which elements of the pathological features might be ameliorated. We compared the effects of the NSAIDs ibuprofen and celecoxib on immunohistological and neurochemical markers at two different ages in APPxPS1 mice using measurements of amyloid plaque deposition, Abeta peptide levels, and neurochemical profiles using magnetic resonance spectroscopy (MRS). At 6 months of age, few neurochemical changes were observed between PSAPP mice and WT mice using MRS. Ibuprofen, but not celecoxib, treatment significantly decreased the Abeta(42/40) ratio in frontal cortex at 6 months, but overall amyloid plaque burden was unchanged. Consistent with prior findings in mouse models, at 17 months of age, there was a decrease in the neuronal markers NAA and glutamate and an increase in the astrocytic markers glutamine and myo-inositol in AD mice compared to WT. Ibuprofen provided significant protection against NAA and glutamate loss. Neither of the drugs significantly affected myo-inositol or glutamine levels. Both ibuprofen and celecoxib lowered plaque burden without a significant effect on Abeta(1-42) levels. NAA levels significantly correlated with plaque burden. These results suggest that selective NSAIDs (ibuprofen and possibly celecoxib) treatment can protect against the neuronal pathology.
Published by Elsevier Inc.
Figures

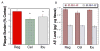
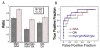



References
-
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. - PubMed
-
- Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR Biomed. 2007;20:216–237. - PubMed
-
- Dedeoglu A, Choi JK, Cormier K, Kowall NW, Jenkins BG. Magnetic resonance spectroscopic analysis of Alzheimer’s disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain Res. 2004;1012:60–65. - PubMed
-
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. - PubMed
-
- in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345:1515–1521. - PubMed

