Evidence that opioids may have toll-like receptor 4 and MD-2 effects
- PMID: 19679181
- PMCID: PMC2788078
- DOI: 10.1016/j.bbi.2009.08.004
Evidence that opioids may have toll-like receptor 4 and MD-2 effects
Abstract
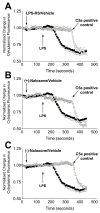
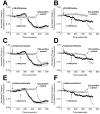
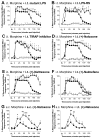
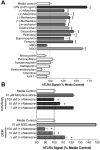
Opioid-induced proinflammatory glial activation modulates wide-ranging aspects of opioid pharmacology including: opposition of acute and chronic opioid analgesia, opioid analgesic tolerance, opioid-induced hyperalgesia, development of opioid dependence, opioid reward, and opioid respiratory depression. However, the mechanism(s) contributing to opioid-induced proinflammatory actions remains unresolved. The potential involvement of toll-like receptor 4 (TLR4) was examined using in vitro, in vivo, and in silico techniques. Morphine non-stereoselectively induced TLR4 signaling in vitro, blocked by a classical TLR4 antagonist and non-stereoselectively by naloxone. Pharmacological blockade of TLR4 signaling in vivo potentiated acute intrathecal morphine analgesia, attenuated development of analgesic tolerance, hyperalgesia, and opioid withdrawal behaviors. TLR4 opposition to opioid actions was supported by morphine treatment of TLR4 knockout mice, which revealed a significant threefold leftward shift in the analgesia dose response function, versus wildtype mice. A range of structurally diverse clinically-employed opioid analgesics was found to be capable of activating TLR4 signaling in vitro. Selectivity in the response was identified since morphine-3-glucuronide, a morphine metabolite with no opioid receptor activity, displayed significant TLR4 activity, whilst the opioid receptor active metabolite, morphine-6-glucuronide, was devoid of such properties. In silico docking simulations revealed ligands bound preferentially to the LPS binding pocket of MD-2 rather than TLR4. An in silico to in vitro prediction model was built and tested with substantial accuracy. These data provide evidence that select opioids may non-stereoselectively influence TLR4 signaling and have behavioral consequences resulting, in part, via TLR4 signaling.
Figures
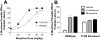

References
-
- Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK. Priming effect of morphine on the production of tumor necrosis factor-alpha by microglia: implications in respiratory burst activity and human immunodeficiency virus-1 expression. J Pharmacol Exp Ther. 1994;269:198–203. - PubMed
-
- Chen ZR, Irvine RJ, Somogyi AA, Bochner F. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 1991;48:2165–71. - PubMed
-
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–43. - PubMed
-
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: Attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–23. - PubMed
-
- Cuschieri J, Bulger E, Billgrin J, Garcia I, Maier RV. Acid sphingomyelinase is required for lipid Raft TLR4 complex formation. Surgical infections. 2007;8:91–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA024044/DA/NIDA NIH HHS/United States
- K02 DA015642/DA/NIDA NIH HHS/United States
- DA017670/DA/NIDA NIH HHS/United States
- T32 GM-065103/GM/NIGMS NIH HHS/United States
- K05 DA024044/DA/NIDA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 DA017670/DA/NIDA NIH HHS/United States
- R01 DE017782/DE/NIDCR NIH HHS/United States
- DE017782/DE/NIDCR NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- DA015642/DA/NIDA NIH HHS/United States
- T32 GM065103/GM/NIGMS NIH HHS/United States
- R01 DA023132/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

