Proton transfer function of carbonic anhydrase: Insights from QM/MM simulations
- PMID: 19679196
- PMCID: PMC6787916
- DOI: 10.1016/j.bbapap.2009.07.026
Proton transfer function of carbonic anhydrase: Insights from QM/MM simulations
Abstract
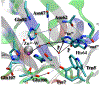
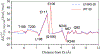
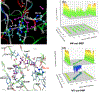
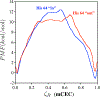
Recent QM/MM analyses of proton transfer function of human carbonic anhydrase II (CAII) are briefly reviewed. The topics include a preliminary analysis of nuclear quadrupole coupling constant calculations for the zinc ion and more detailed analyses of microscopic pK(a) of the zinc-bound water and free energy profile for the proton transfer. From a methodological perspective, our results emphasize that performing sufficient sampling is essential to the calculation of all these quantities, which reflects the well solvated nature of CAII active site. From a mechanistic perspective, our analyses highlight the importance of electrostatics in shaping the energetics and kinetics of proton transfer in CAII for its function. We argue that once the pK(a) for the zinc-bound water is modulated to be in the proper range (approximately 7.0), proton transfer through a relatively well solvated cavity towards/from the protein surface (His64) does not require any major acceleration. Therefore, although structural details like the length of the water wire between the donor and acceptor groups still may make a non-negligible contribution, our computational results and the framework of analysis suggest that the significance of such "fine-tuning" is likely secondary to the modulation of pK(a) of the zinc-bound water. We encourage further experimental analysis with mutation of (charged) residues not in the immediate neighborhood of the zinc ion to quantitatively test this electrostatics based framework; in particular, Phi analysis based on these mutations may shed further light into the relative importance of the classical Grotthus mechanism and the "proton hole" pathway that we have proposed recently for CAII.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Proton transfer in carbonic anhydrase is controlled by electrostatics rather than the orientation of the acceptor.Biochemistry. 2008 Feb 26;47(8):2369-78. doi: 10.1021/bi701950j. Epub 2008 Feb 2. Biochemistry. 2008. PMID: 18247480
-
Proton transfer in catalysis and the role of proton shuttles in carbonic anhydrase.Biochim Biophys Acta. 2010 Feb;1804(2):422-6. doi: 10.1016/j.bbapap.2009.08.003. Epub 2009 Aug 11. Biochim Biophys Acta. 2010. PMID: 19679199 Free PMC article.
-
Disruption of the active site solvent network in carbonic anhydrase II decreases the efficiency of proton transfer.Biochemistry. 1996 Dec 24;35(51):16421-8. doi: 10.1021/bi961786+. Biochemistry. 1996. PMID: 8987973
-
Computer simulation of the initial proton transfer step in human carbonic anhydrase I.J Mol Biol. 1992 Mar 5;224(1):7-14. doi: 10.1016/0022-2836(92)90572-2. J Mol Biol. 1992. PMID: 1312606 Review.
-
Proton transport in carbonic anhydrase: Insights from molecular simulation.Biochim Biophys Acta. 2010 Feb;1804(2):332-41. doi: 10.1016/j.bbapap.2009.09.006. Epub 2009 Sep 16. Biochim Biophys Acta. 2010. PMID: 19765680 Free PMC article. Review.
Cited by
-
Parametrization of DFTB3/3OB for magnesium and zinc for chemical and biological applications.J Phys Chem B. 2015 Jan 22;119(3):1062-82. doi: 10.1021/jp506557r. Epub 2014 Sep 16. J Phys Chem B. 2015. PMID: 25178644 Free PMC article.
-
Parameterization of DFTB3/3OB for Sulfur and Phosphorus for Chemical and Biological Applications.J Chem Theory Comput. 2014 Apr 8;10(4):1518-1537. doi: 10.1021/ct401002w. Epub 2014 Mar 12. J Chem Theory Comput. 2014. PMID: 24803865 Free PMC article.
-
An Update on the Metabolic Roles of Carbonic Anhydrases in the Model Alga Chlamydomonas reinhardtii.Metabolites. 2018 Mar 13;8(1):22. doi: 10.3390/metabo8010022. Metabolites. 2018. PMID: 29534024 Free PMC article. Review.
-
Benchmark Study of the SCC-DFTB Approach for a Biomolecular Proton Channel.J Chem Theory Comput. 2014 Jan 14;10(1):451-462. doi: 10.1021/ct400832r. J Chem Theory Comput. 2014. PMID: 25104919 Free PMC article.
-
DFTB3: Extension of the self-consistent-charge density-functional tight-binding method (SCC-DFTB).J Chem Theory Comput. 2012 Apr 10;7(4):931-948. doi: 10.1021/ct100684s. J Chem Theory Comput. 2012. PMID: 23204947 Free PMC article.
References
-
- Silverman DN, McKenna R, Solvent mediated proton transfer in catalysis by carbonic anhydrase, Acc. Chem. Res 40 (2007) 669–675. - PubMed
-
- Ellis PD, Lipton AS, Low-temperature solid-state NMR spectroscopy. A strategy for the direct observation of quadrupolar nuclides of biological interest, Annu. Rep. NMR Spectro 60 (2007) 1–38.
-
- McDermott A, Polenova T, Solid state NMR: new tools for insight into enzyme function, Curr. Opin. Struct. Biol 17 (2007) 617–622. - PubMed
-
- Kaufmann EN, Vianden RJ, The electric field gradient in noncubic metals, Rev. Mod. Phys 51 (1979) 161–214.
-
- Lipton AS, Heck RW, Ellis PD, Zinc Solid-State NMR spectroscopy of human Carbonic Anhydrase: Implications for the enzymatic mechanism, J. Am. Chem. Soc 126 (2004) 4735–4739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

