The effect of variation in expression of the candidate dyslexia susceptibility gene homolog Kiaa0319 on neuronal migration and dendritic morphology in the rat
- PMID: 19679544
- PMCID: PMC2837091
- DOI: 10.1093/cercor/bhp154
The effect of variation in expression of the candidate dyslexia susceptibility gene homolog Kiaa0319 on neuronal migration and dendritic morphology in the rat
Abstract
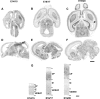
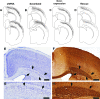
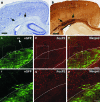
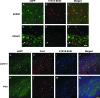
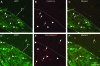
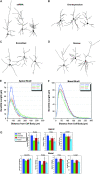
We investigated the postnatal effects of embryonic knockdown and overexpression of the candidate dyslexia gene homolog Kiaa0319. We used in utero electroporation to transfect cells in E15/16 rat neocortical ventricular zone with either 1) small hairpin RNA (shRNA) vectors targeting Kiaa0319, 2) a KIAA0319 expression construct, 3) Kiaa0319 shRNA along with KIAA0319 expression construct ("rescue"), or 4) a scrambled version of Kiaa0319 shRNA. Knockdown, but not overexpression, of Kiaa0319 resulted in periventricular heterotopias that contained large numbers of both transfected and non-transfected neurons. This suggested that Kiaa0319 shRNA disrupts neuronal migration by cell autonomous as well as non-cell autonomous mechanisms. Of the Kiaa0319 shRNA-transfected neurons that migrated into the cortical plate, most migrated to their appropriate lamina. In contrast, neurons transfected with the KIAA0319 expression vector attained laminar positions subjacent to their expected positions. Neurons transfected with Kiaa0319 shRNA exhibited apical, but not basal, dendrite hypertrophy, which was rescued by overexpression of KIAA0319. The results provide additional supportive evidence linking candidate dyslexia susceptibility genes to migrational disturbances during brain development, and extends the role of Kiaa0319 to include growth and differentiation of dendrites.
Figures



References
-
- Allen KM, Gleeson JG, Shoup SM, Walsh CA. A YAC contig in Xq22.3-q23, from DXS287 to DXS8088, spanning the brain- specific genes doublecortin (DCX) and PAK3. Genomics. 1998;52:214–218. - PubMed
-
- Anderson S, Marin O, Horn C, Jennings K, Rubenstein J. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
-
- Andrews W, Barber M, Hernadez-Miranda LR, Xian J, Rakic S, Sundaresan V, Rabbitts TH, Pannell R, Rabbitts P, Thompson H, et al. The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev Biol. 2008;313:648–658. - PubMed
-
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. - PubMed
-
- Banerjee M, Worth D, Prowse DM, Nikolic M. Pak1 phosphorylation on t212 affects microtubules in cells undergoing mitosis. Curr Biol. 2002;12:1233–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

