Formation of ArF from LPdAr(F): catalytic conversion of aryl triflates to aryl fluorides
- PMID: 19679769
- PMCID: PMC3038120
- DOI: 10.1126/science.1178239
Formation of ArF from LPdAr(F): catalytic conversion of aryl triflates to aryl fluorides
Erratum in
- Science. 2010 Jul 2;329(5987):33
Abstract
Despite increasing pharmaceutical importance, fluorinated aromatic organic molecules remain difficult to synthesize. Present methods require either harsh reaction conditions or highly specialized reagents, making the preparation of complex fluoroarenes challenging. Thus, the development of general methods for their preparation that overcome the limitations of those techniques currently in use is of great interest. We have prepared [LPd(II)Ar(F)] complexes, where L is a biaryl monophosphine ligand and Ar is an aryl group, and identified conditions under which reductive elimination occurs to form an Ar-F bond. On the basis of these results, we have developed a catalytic process that converts aryl bromides and aryl triflates into the corresponding fluorinated arenes by using simple fluoride salts. We expect this method to allow the introduction of fluorine atoms into advanced, highly functionalized intermediates.
Figures
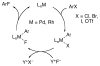


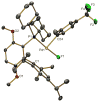

Comment in
-
Chemistry. A new departure in fluorination chemistry.Science. 2009 Sep 25;325(5948):1630-1. doi: 10.1126/science.1179671. Science. 2009. PMID: 19779178 No abstract available.
References
-
- Müller K, Faeh C, Diederich F. Science. 2007;317:1881. - PubMed
-
- Böhm HJ, Banner D, Bendels S, Kansy M, Kuhn B, Müller K, Obst-Sander U, Stahl M. ChemBioChem. 2004;5:637. - PubMed
-
- Gerebtzoff G, Li-Blatter X, Fischer H, Frentzel A, Seelig A. ChemBioChem. 2004;5:676. - PubMed
-
- Jeschke P. ChemBioChem. 2004;5:570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

