Thymus-specific deletion of insulin induces autoimmune diabetes
- PMID: 19680229
- PMCID: PMC2750011
- DOI: 10.1038/emboj.2009.212
Thymus-specific deletion of insulin induces autoimmune diabetes
Abstract
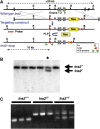
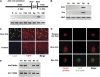
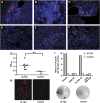
Insulin expression in the thymus has been implicated in regulating the negative selection of autoreactive T cells and in mediating the central immune tolerance towards pancreatic beta-cells. To further explore the function of this ectopic insulin expression, we knocked out the mouse Ins2 gene specifically in the Aire-expressing medullary thymic epithelial cells (mTECs), without affecting its expression in the beta-cells. When further crossed to the Ins1 knockout background, both male and female pups (designated as ID-TEC mice for insulin-deleted mTEC) developed diabetes spontaneously around 3 weeks after birth. beta-cell-specific autoimmune destruction was observed, as well as islet-specific T cell infiltration. The presence of insulin-specific effector T cells was shown using ELISPOT assays and adoptive T cell transfer experiments. Results from thymus transplantation experiments proved further that depletion of Ins2 expression in mTECs was sufficient to break central tolerance and induce anti-insulin autoimmunity. Our observations may explain the rare cases of type 1 diabetes onset in very young children carrying diabetes-resistant HLA class II alleles. ID-TEC mice could serve as a new model for studying this pathology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

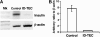



Comment in
-
Insulin teaches a new lesson in tolerance.EMBO J. 2009 Sep 16;28(18):2687-8. doi: 10.1038/emboj.2009.251. EMBO J. 2009. PMID: 19759522 Free PMC article. No abstract available.
References
-
- Abiru N, Maniatis AK, Yu L, Miao D, Moriyama H, Wegmann D, Eisenbarth GS (2001) Peptide and major histocompatibility complex-specific breaking of humoral tolerance to native insulin with the B9-23 peptide in diabetes-prone and normal mice. Diabetes 50: 1274–1281 - PubMed
-
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D (2005) The cellular mechanism of Aire control of T cell tolerance. Immunity 23: 227–239 - PubMed
-
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D (2002) Projection of an immunological self shadow within the thymus by the Aire protein. Science 298: 1395–1401 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

