Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model
- PMID: 19680448
- PMCID: PMC2718809
- DOI: 10.1371/journal.ppat.1000551
Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model
Abstract
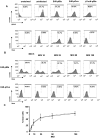
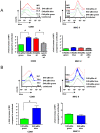
Yersinia enterocolitica (Ye) evades the immune system of the host by injection of Yersinia outer proteins (Yops) via a type three secretion system into host cells. In this study, a reporter system comprising a YopE-beta-lactamase hybrid protein and a fluorescent staining sensitive to beta-lactamase cleavage was used to track Yop injection in cell culture and in an experimental Ye mouse infection model. Experiments with GD25, GD25-beta1A, and HeLa cells demonstrated that beta1-integrins and RhoGTPases play a role for Yop injection. As demonstrated by infection of splenocyte suspensions in vitro, injection of Yops appears to occur randomly into all types of leukocytes. In contrast, upon infection of mice, Yop injection was detected in 13% of F4/80(+), 11% of CD11c(+), 7% of CD49b(+), 5% of Gr1(+) cells, 2.3% of CD19(+), and 2.6% of CD3(+) cells. Taking the different abundance of these cell types in the spleen into account, the highest total number of Yop-injected cells represents B cells, particularly CD19(+)CD21(+)CD23(+) follicular B cells, followed by neutrophils, dendritic cells, and macrophages, suggesting a distinct cellular tropism of Ye. Yop-injected B cells displayed a significantly increased expression of CD69 compared to non-Yop-injected B cells, indicating activation of these cells by Ye. Infection of IFN-gammaR (receptor)- and TNFRp55-deficient mice resulted in increased numbers of Yop-injected spleen cells for yet unknown reasons. The YopE-beta-lactamase hybrid protein reporter system provides new insights into the modulation of host cell and immune responses by Ye Yops.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Smego RA, Frean J, Koornhof HJ. Yersiniosis I: microbiological and clinicoepidemiological aspects of plague and non-plague Yersinia infections. Eur J Clin Microbiol Infect Dis. 1999;18:1–15. - PubMed
-
- Koornhof HJ, Smego RA, Jr, Nicol M. Yersiniosis. II: The pathogenesis of Yersinia infections. Eur J Clin Microbiol Infect Dis. 1999;18:87–112. - PubMed
-
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

