A model for cortical rewiring following deafferentation and focal stroke
- PMID: 19680468
- PMCID: PMC2726035
- DOI: 10.3389/neuro.10.010.2009
A model for cortical rewiring following deafferentation and focal stroke
Abstract
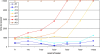
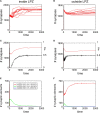
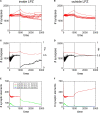
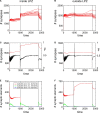
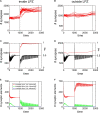
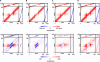
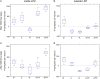
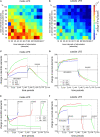
It is still unclear to what extent structural plasticity in terms of synaptic rewiring is the cause for cortical remapping after a lesion. Recent two-photon laser imaging studies demonstrate that synaptic rewiring is persistent in the adult brain and is dramatically increased following brain lesions or after a loss of sensory input (cortical deafferentation). We use a recurrent neural network model to study the time course of synaptic rewiring following a peripheral lesion. For this, we represent axonal and dendritic elements of cortical neurons to model synapse formation, pruning and synaptic rewiring. Neurons increase and decrease the number of axonal and dendritic elements in an activity-dependent fashion in order to maintain their activity in a homeostatic equilibrium. In this study we demonstrate that synaptic rewiring contributes to neuronal homeostasis during normal development as well as following lesions. We show that networks in homeostasis, which can therefore be considered as adult networks, are much less able to compensate for a loss of input. Interestingly, we found that paused stimulation of the networks are much more effective promoting reorganization than continuous stimulation. This can be explained as neurons quickly adapt to this stimulation whereas pauses prevents a saturation of the positive stimulation effect. These findings may suggest strategies for improving therapies in neurologic rehabilitation.
Keywords: axonal sprouting; cortical remapping; homeostasis; lesion-induced plasticity; neurological rehabilitation; neuronal network model; structural plasticity; synaptogenesis.
Figures

Similar articles
-
Homeostatic structural plasticity can account for topology changes following deafferentation and focal stroke.Front Neuroanat. 2014 Oct 16;8:115. doi: 10.3389/fnana.2014.00115. eCollection 2014. Front Neuroanat. 2014. PMID: 25360087 Free PMC article.
-
A simple rule for dendritic spine and axonal bouton formation can account for cortical reorganization after focal retinal lesions.PLoS Comput Biol. 2013;9(10):e1003259. doi: 10.1371/journal.pcbi.1003259. Epub 2013 Oct 10. PLoS Comput Biol. 2013. PMID: 24130472 Free PMC article.
-
Growth rules for the repair of Asynchronous Irregular neuronal networks after peripheral lesions.PLoS Comput Biol. 2021 Jun 1;17(6):e1008996. doi: 10.1371/journal.pcbi.1008996. eCollection 2021 Jun. PLoS Comput Biol. 2021. PMID: 34061830 Free PMC article.
-
Integrated technology for evaluation of brain function and neural plasticity.Phys Med Rehabil Clin N Am. 2004 Feb;15(1):263-306. doi: 10.1016/s1047-9651(03)00124-4. Phys Med Rehabil Clin N Am. 2004. PMID: 15029909 Review.
-
Sensory experience and cortical rewiring.Neuroscientist. 2010 Apr;16(2):186-98. doi: 10.1177/1073858409343961. Epub 2009 Oct 1. Neuroscientist. 2010. PMID: 19801372 Review.
Cited by
-
Using theoretical models to analyse neural development.Nat Rev Neurosci. 2011 Jun;12(6):311-26. doi: 10.1038/nrn3031. Epub 2011 May 18. Nat Rev Neurosci. 2011. PMID: 21587288 Review.
-
Pathological neural attractor dynamics in slowly growing gliomas supports an optimal time frame for white matter plasticity.PLoS One. 2013 Jul 26;8(7):e69798. doi: 10.1371/journal.pone.0069798. Print 2013. PLoS One. 2013. PMID: 23922804 Free PMC article.
-
Bioscaffold-Induced Brain Tissue Regeneration.Front Neurosci. 2019 Nov 7;13:1156. doi: 10.3389/fnins.2019.01156. eCollection 2019. Front Neurosci. 2019. PMID: 31787865 Free PMC article. Review.
-
Mesoscopic cortical network reorganization during recovery of optic nerve injury in GCaMP6s mice.Sci Rep. 2020 Dec 8;10(1):21472. doi: 10.1038/s41598-020-78491-z. Sci Rep. 2020. PMID: 33293617 Free PMC article.
-
Resolving inconsistent effects of tDCS on learning using a homeostatic structural plasticity model.Front Netw Physiol. 2025 Jul 7;5:1565802. doi: 10.3389/fnetp.2025.1565802. eCollection 2025. Front Netw Physiol. 2025. PMID: 40692813 Free PMC article.
References
LinkOut - more resources
Full Text Sources

