Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression
- PMID: 19680549
- PMCID: PMC2721630
- DOI: 10.1371/journal.pone.0006654
Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression
Abstract
Inactivating mutations in the p53 tumor suppressor gene occur often in the progression of human cancers. p53 inhibits the outgrowth of nascent cancer cells through anti-proliferative actions (including induction of apoptosis or senescence). To test p53 tumor suppressor functions in a novel experimental context, we somatically deleted both p53 alleles in multiple tissues of mice at various ages. Mice homozygously deleted for p53 at 3 months of age showed a longer tumor latency compared to mice deleted for p53 at 6 and 12 months of age. These results are consistent with a model in which tissues accumulate oncogenically activated cells with age and these are held in check by wildtype p53. We also deleted p53 before, concurrent with, and after treatment of mice with ionizing radiation (IR). The absence or presence of p53 during IR treatment had no effect on radiation-induced lymphoma latency, confirming that the immediate p53 damage response was irrelevant for cancer prevention. Even the presence of wildtype p53 for up to four weeks post-IR provided no protection against early lymphoma incidence, indicating that long term maintenance of functional p53 is critical for preventing the emergence of a cancer. These experiments indicate that sustained p53 anti-oncogenic function acts as a final or near final line of defense preventing progression of oncogenically activated cells to malignant tumors.
Conflict of interest statement
Figures
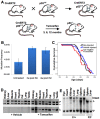
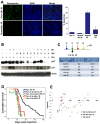
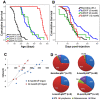
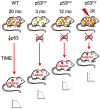
References
-
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. - PubMed
-
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. - PubMed
-
- Soussi T, Wiman KG. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell. 2007;12:303–312. - PubMed
-
- Macleod K. Tumor suppressor genes. Curr Opin Genet Dev. 2000;10:81–93. - PubMed
-
- Blondal JA, Benchimol S. The role of p53 in tumor progression. Semin Cancer Biol. 1994;5:177–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

