Immature and maturation-resistant human dendritic cells generated from bone marrow require two stimulations to induce T cell anergy in vitro
- PMID: 19680551
- PMCID: PMC2721636
- DOI: 10.1371/journal.pone.0006645
Immature and maturation-resistant human dendritic cells generated from bone marrow require two stimulations to induce T cell anergy in vitro
Abstract
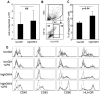
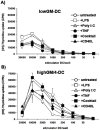
Immature dendritic cells (DC) represent potential clinical tools for tolerogenic cellular immunotherapy in both transplantation and autoimmunity. A major drawback in vivo is their potential to mature during infections or inflammation, which would convert their tolerogenicity into immunogenicity. The generation of immature DC from human bone marrow (BM) by low doses of GM-CSF (lowGM) in the absence of IL-4 under GMP conditions create DC resistant to maturation, detected by surface marker expression and primary stimulation by allogeneic T cells. This resistence could not be observed for BM-derived DC generated with high doses of GM-CSF plus IL-4 (highGM/4), although both DC types induced primary allogeneic T cell anergy in vitro. The estabishment of the anergic state requires two subsequent stimulations by immature DC. Anergy induction was more profound with lowGM-DC due to their maturation resistance. Together, we show the generation of immature, maturation-resistant lowGM-DC for potential clinical use in transplant rejection and propose a two-step-model of T cell anergy induction by immature DC.
Conflict of interest statement
Figures



Similar articles
-
Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro.Eur J Immunol. 2000 Apr;30(4):1048-52. doi: 10.1002/(SICI)1521-4141(200004)30:4<1048::AID-IMMU1048>3.0.CO;2-W. Eur J Immunol. 2000. PMID: 10760792
-
Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo.Eur J Immunol. 2000 Jul;30(7):1813-22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. Eur J Immunol. 2000. PMID: 10940870
-
Dendritic cells generated with Flt3L and exposed to apoptotic cells lack induction of T cell anergy and Foxp3⁺ regulatory T cell conversion in vitro.Immunobiology. 2014 Mar;219(3):230-40. doi: 10.1016/j.imbio.2013.10.006. Epub 2013 Oct 24. Immunobiology. 2014. PMID: 24252473
-
Bone marrow-derived immature dendritic cells prime in vivo alloreactive T cells for interleukin-4-dependent rejection of major histocompatibility complex class II antigen-disparate cardiac allograft.Transplantation. 2003 Feb 15;75(3):407-13. doi: 10.1097/01.TP.0000044172.19087.22. Transplantation. 2003. PMID: 12589166
-
Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets.Hum Immunol. 2002 Dec;63(12):1156-63. doi: 10.1016/s0198-8859(02)00754-1. Hum Immunol. 2002. PMID: 12480259 Review.
Cited by
-
Mast cell mediators in tolerance.Curr Opin Immunol. 2010 Oct;22(5):643-8. doi: 10.1016/j.coi.2010.08.015. Epub 2010 Sep 29. Curr Opin Immunol. 2010. PMID: 20884193 Free PMC article. Review.
-
Tolerogenic dendritic cells and negative vaccination in transplantation: from rodents to clinical trials.Front Immunol. 2012 Aug 9;3:218. doi: 10.3389/fimmu.2012.00218. eCollection 2012. Front Immunol. 2012. PMID: 22908013 Free PMC article.
-
Dendritic cell-based approaches for therapeutic immune regulation in solid-organ transplantation.J Transplant. 2013;2013:761429. doi: 10.1155/2013/761429. Epub 2013 Oct 24. J Transplant. 2013. PMID: 24307940 Free PMC article. Review.
-
Gram-negative enterobacteria induce tolerogenic maturation in dexamethasone conditioned dendritic cells.PLoS One. 2012;7(12):e52456. doi: 10.1371/journal.pone.0052456. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300676 Free PMC article.
-
Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy.J Interferon Cytokine Res. 2015 Aug;35(8):585-99. doi: 10.1089/jir.2014.0149. Epub 2015 Mar 24. J Interferon Cytokine Res. 2015. PMID: 25803788 Free PMC article. Review.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7:650–654. - PubMed
-
- Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

