Sterol Biosynthesis Pathway as Target for Anti-trypanosomatid Drugs
- PMID: 19680554
- PMCID: PMC2721973
- DOI: 10.1155/2009/642502
Sterol Biosynthesis Pathway as Target for Anti-trypanosomatid Drugs
Abstract
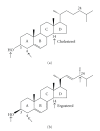
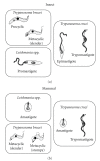
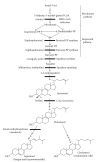
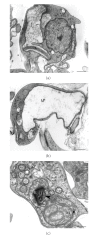
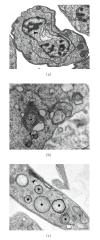
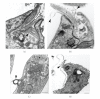
Sterols are constituents of the cellular membranes that are essential for their normal structure and function. In mammalian cells, cholesterol is the main sterol found in the various membranes. However, other sterols predominate in eukaryotic microorganisms such as fungi and protozoa. It is now well established that an important metabolic pathway in fungi and in members of the Trypanosomatidae family is one that produces a special class of sterols, including ergosterol, and other 24-methyl sterols, which are required for parasitic growth and viability, but are absent from mammalian host cells. Currently, there are several drugs that interfere with sterol biosynthesis (SB) that are in use to treat diseases such as high cholesterol in humans and fungal infections. In this review, we analyze the effects of drugs such as (a) statins, which act on the mevalonate pathway by inhibiting HMG-CoA reductase, (b) bisphosphonates, which interfere with the isoprenoid pathway in the step catalyzed by farnesyl diphosphate synthase, (c) zaragozic acids and quinuclidines, inhibitors of squalene synthase (SQS), which catalyzes the first committed step in sterol biosynthesis, (d) allylamines, inhibitors of squalene epoxidase, (e) azoles, which inhibit C14alpha-demethylase, and (f) azasterols, which inhibit Delta(24(25))-sterol methyltransferase (SMT). Inhibition of this last step appears to have high selectivity for fungi and trypanosomatids, since this enzyme is not found in mammalian cells. We review here the IC50 values of these various inhibitors, their effects on the growth of trypanosomatids (both in axenic cultures and in cell cultures), and their effects on protozoan structural organization (as evaluted by light and electron microscopy) and lipid composition. The results show that the mitochondrial membrane as well as the membrane lining the protozoan cell body and flagellum are the main targets. Probably as a consequence of these primary effects, other important changes take place in the organization of the kinetoplast DNA network and on the protozoan cell cycle. In addition, apoptosis-like and autophagic processes induced by several of the inhibitors tested led to parasite death.
Figures








References
-
- Fagundes LJM. Estudo de esteróis e lipídeos totais em Leptomonas pessoai cultivada a 28°C e 37°C. Rio de Janeiro, Brazil: Instituto de Microbiologia da Universidade Federal do Rio de Janeiro; 1974. M.S. thesis.
-
- de Souza W, Sant'anna C, Cunha-e-Silva NL. Electron microscopy and cytochemistry analysis of the endocytic pathway of pathogenic protozoa. Progress in Histochemistry and Cytochemistry. 2009;44(2):67–124. - PubMed
-
- Soares MJ, de Souza W. Endocytosis of gold-labeled proteins and LDL by Trypanosoma cruzi . Parasitology Research. 1991;77(6):461–468. - PubMed
-
- Urbina JA. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology. 1997;114(supplement 1):S91–S99. - PubMed
-
- Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML, Goad LJ. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Molecular and Biochemical Parasitology. 2003;126(2):129–142. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

