Characterizing dispersion in microfluidic channels
- PMID: 19680578
- PMCID: PMC2814782
- DOI: 10.1039/b822948c
Characterizing dispersion in microfluidic channels
Abstract
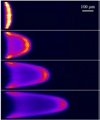
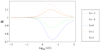
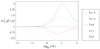
Dispersion or spreading of analyte bands is a barrier to achieving high resolution in microfluidic separations. The role of dispersion in separations is reviewed with emphasis on metrics, sources and common principles of analysis. Three sources of dispersion (a) inhomogeneous flow fields, (b) solute wall interactions and (c) force fields normal to channel walls are studied in detail. Microfluidic and nanofluidic applications to capillary electrophoresis, chromatography and field-flow fractionation, that are subject to one or more of these three physical processes under standard, unintentional or novel operating conditions, are discussed.
Figures





References
-
- Giddings J. Dynamics of Chromatography, Part I, Principles and Theory. New York, U.S.A: Marcel Dekker, Inc.; 1965.
-
- Taylor G. Proc. Roy. Soc. A. 1953;219:186.
-
- Bello M, Rezzonico R, Righetti P. Science. 1994;266:773. - PubMed
-
- Brenner H, Edwards D. Macrotransport Processes. Butterworth-Heinemann; 1993.
-
- Bloechle B. Ph.D. thesis. University of Colorado; 2001.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

