Celiac disease IgA modulates vascular permeability in vitro through the activity of transglutaminase 2 and RhoA
- PMID: 19680746
- PMCID: PMC11115502
- DOI: 10.1007/s00018-009-0116-1
Celiac disease IgA modulates vascular permeability in vitro through the activity of transglutaminase 2 and RhoA
Abstract
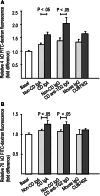
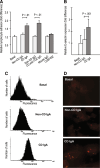
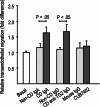
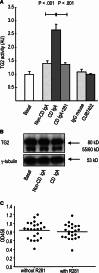
Celiac disease is characterized by the presence of specific autoantibodies targeted against transglutaminase 2 (TG2) in untreated patients' serum and at their production site in the small-bowel mucosa below the basement membrane and around the blood vessels. As these autoantibodies have biological activity in vitro, such as inhibition of angiogenesis, we studied if they might also modulate the endothelial barrier function. Our results show that celiac disease patient autoantibodies increase endothelial permeability for macromolecules, and enhance the binding of lymphocytes to the endothelium and their transendothelial migration when compared to control antibodies in an endothelial cell-based in vitro model. We also demonstrate that these effects are mediated by increased activities of TG2 and RhoA. Since the small bowel mucosal endothelium serves as a "gatekeeper" in inflammatory processes, the disease-specific autoantibodies targeted against TG2 could thus contribute to the pathogenic cascade of celiac disease by increasing blood vessel permeability.
Figures

Similar articles
-
Celiac disease patient IgA antibodies induce endothelial adhesion and cell polarization defects via extracellular transglutaminase 2.Cell Mol Life Sci. 2014 Apr;71(7):1315-26. doi: 10.1007/s00018-013-1455-5. Epub 2013 Aug 28. Cell Mol Life Sci. 2014. PMID: 23982754 Free PMC article.
-
Coeliac disease-specific autoantibodies targeted against transglutaminase 2 disturb angiogenesis.Clin Exp Immunol. 2008 Apr;152(1):111-9. doi: 10.1111/j.1365-2249.2008.03600.x. Epub 2008 Feb 14. Clin Exp Immunol. 2008. PMID: 18279443 Free PMC article.
-
High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions.Nat Med. 2012 Feb 26;18(3):441-5. doi: 10.1038/nm.2656. Nat Med. 2012. PMID: 22366952 Free PMC article.
-
Transglutaminase 2 and Transglutaminase 2 Autoantibodies in Celiac Disease: a Review.Clin Rev Allergy Immunol. 2019 Aug;57(1):23-38. doi: 10.1007/s12016-016-8557-4. Clin Rev Allergy Immunol. 2019. PMID: 27263022 Review.
-
Anti-type 2 transglutaminase antibodies as modulators of type 2 transglutaminase functions: a possible pathological role in celiac disease.Cell Mol Life Sci. 2018 Nov;75(22):4107-4124. doi: 10.1007/s00018-018-2902-0. Epub 2018 Aug 22. Cell Mol Life Sci. 2018. PMID: 30136165 Free PMC article. Review.
Cited by
-
Thioredoxin is involved in endothelial cell extracellular transglutaminase 2 activation mediated by celiac disease patient IgA.PLoS One. 2013 Oct 9;8(10):e77277. doi: 10.1371/journal.pone.0077277. eCollection 2013. PLoS One. 2013. PMID: 24130874 Free PMC article.
-
Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder.Front Immunol. 2020 Jul 8;11:1374. doi: 10.3389/fimmu.2020.01374. eCollection 2020. Front Immunol. 2020. PMID: 32733456 Free PMC article. Review.
-
Retinal and choroidal vascular changes in newly diagnosed celiac disease: An optical coherence tomography angiography study.Indian J Ophthalmol. 2022 Mar;70(3):866-870. doi: 10.4103/ijo.IJO_1009_21. Indian J Ophthalmol. 2022. PMID: 35225533 Free PMC article.
-
Cardiovascular involvement in celiac disease.World J Cardiol. 2017 Aug 26;9(8):652-666. doi: 10.4330/wjc.v9.i8.652. World J Cardiol. 2017. PMID: 28932354 Free PMC article. Review.
-
Cardiomyopathy in Celiac Disease: A Systematic Review.J Clin Med. 2024 Feb 12;13(4):1045. doi: 10.3390/jcm13041045. J Clin Med. 2024. PMID: 38398359 Free PMC article. Review.
References
-
- Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol. 2001;166:4170–4176. - PubMed
-
- Salmi TT, Collin P, Korponay-Szabo IR, Laurila K, Partanen J, Huhtala H, Kiraly R, Lorand L, Reunala T, Maki M, Kaukinen K. Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut. 2006;55:1746–1753. doi: 10.1136/gut.2005.071514. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

