Mast cell degranulation breaks peripheral tolerance
- PMID: 19681828
- PMCID: PMC3808998
- DOI: 10.1111/j.1600-6143.2009.02755.x
Mast cell degranulation breaks peripheral tolerance
Abstract
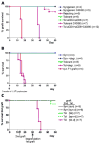
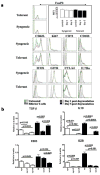
Mast cells (MC) have been shown to mediate regulatory T-cell (T(reg))-dependent, peripheral allograft tolerance in both skin and cardiac transplants. Furthermore, T(reg) have been implicated in mitigating IgE-mediated MC degranulation, establishing a dynamic, reciprocal relationship between MC and T(reg) in controlling inflammation. In an allograft tolerance model, it is now shown that intragraft or systemic MC degranulation results in the transient loss of T(reg) suppressor activities with the acute, T-cell dependent rejection of established, tolerant allografts. Upon degranulation, MC mediators can be found in the skin, T(reg) rapidly leave the graft, MC accumulate in the regional lymph node and the T(reg) are impaired in the expression of suppressor molecules. Such a dramatic reversal of T(reg) function and tissue distribution by MC degranulation underscores how allergy may causes the transient breakdown of peripheral tolerance and episodes of acute T-cell inflammation.
Figures




Comment in
-
MASTering Treg function to promote tolerance.Am J Transplant. 2009 Oct;9(10):2209-10. doi: 10.1111/j.1600-6143.2009.02810.x. Am J Transplant. 2009. PMID: 19764946 No abstract available.
-
Literature watch: Implications for transplantation. Mast cells: inflammatory, immunoregulatory or something in between?Am J Transplant. 2012 Sep;12(9):2265. doi: 10.1111/j.1600-6143.2012.04256.x. Am J Transplant. 2012. PMID: 22925181 No abstract available.
References
-
- Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19(1):31–38. - PubMed
-
- Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cell in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. - PubMed
-
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8(10):1095–1104. - PubMed
-
- Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol. 2006;176(7):4141–4146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

