Preclinical evaluation of a monoclonal antibody targeting the epidermal growth factor receptor as a radioimmunodiagnostic and radioimmunotherapeutic agent
- PMID: 19681874
- PMCID: PMC2765311
- DOI: 10.1111/j.1476-5381.2009.00327.x
Preclinical evaluation of a monoclonal antibody targeting the epidermal growth factor receptor as a radioimmunodiagnostic and radioimmunotherapeutic agent
Abstract
Background and purpose: The studies described here are the first to evaluate the in vitro and in vivo properties of (111)In-CHX-A''-panitumumab for radioimmunotherapy (alpha- and beta(-)-emitters) and radioimmunoimaging (single photon emission computed tomography and positron emission tomography).
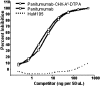
Experimental approach: Twenty-seven human carcinoma cell lines were analysed for expression of epidermal growth factor receptors by flow cytometry. Panitumumab was conjugated with CHX-A''-DTPA (diethylenetriamine-pentaacetic acid) and radiolabelled with (111)In. Immunoreactivity of the CHX-A''-DTPA-panitumumab and (111)In-CHX-A''-DTPA-panitumumab was evaluated by radioimmunoassays. Tumour targeting was determined in vivo by direct quantitation of tumour and normal tissues and by gamma-scintigraphy.
Key results: For 26 of 27 human tumour cell lines, 95% of the cells expressed epidermal growth factor receptors over a range of intensity. Immunoreactivity of panitumumab was retained after modification with CHX-A''-DTPA. Radiolabelling of the immunoconjugate with (111)In was efficient with a specific activity of 19.5 +/- 8.9 mCi.mg(-1) obtained. Immunoreactivity and specificity of binding of the (111)In-panitumumab was shown with A431 cells. Tumour targeting by (111)In-panitumumab was demonstrated in athymic mice bearing A431, HT-29, LS-174T, SHAW or SKOV-3 s.c. xenografts with little uptake observed in normal tissues. The (111)In-panitumumab was also evaluated in non-tumour-bearing mice. Pharmacokinetic studies compared the plasma retention time of the (111)In-panitumumab in both non-tumour-bearing and A431 tumour-bearing mice. Tumour targeting was also visualized by gamma-scintigraphy.
Conclusions and implications: Panitumumab can be efficiently radiolabelled with (111)In with high labelling yields. Based on the efficiency in tumour targeting and low normal tissue uptake, panitumumab may be an effective targeting component for radioimmunodiagnostic and radioimmunotherapeutic applications.
Figures



References
-
- Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7(Suppl. 4):2–8. - PubMed
-
- Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. - PubMed
-
- Carrasquillo JA, Sugarbaker P, Colcher D, Reynolds JC, Esteban J, Bryant G, et al. Radioimmunoscintigraphy of colon cancer with iodine-131-labeled B72.3 monoclonal antibody. J Nucl Med. 1988;29:1022–1030. - PubMed
-
- Cohenuram M, Saif MW. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs. 2007;18:7–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

