Identification of Chlamydia trachomatis CT621, a protein delivered through the type III secretion system to the host cell cytoplasm and nucleus
- PMID: 19682078
- PMCID: PMC2784215
- DOI: 10.1111/j.1574-695X.2009.00581.x
Identification of Chlamydia trachomatis CT621, a protein delivered through the type III secretion system to the host cell cytoplasm and nucleus
Abstract
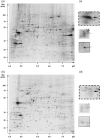
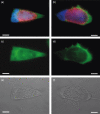
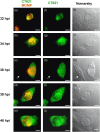
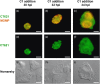
Chlamydiae are obligate intracellular bacteria, developing inside host cells within chlamydial inclusions. From these inclusions, the chlamydiae secrete proteins into the host cell cytoplasm. A pathway through which secreted proteins can be delivered is the type III secretion system (T3SS). The T3SS is common to several gram-negative bacteria and the secreted proteins serve a variety of functions often related to the modulation of host signalling. To identify new potentially secreted proteins, the cytoplasm was extracted from Chlamydia trachomatis L2-infected HeLa cells, and two-dimensional polyacrylamide gel electrophoresis profiles of [(35)S]-labelled chlamydial proteins from this extract were compared with profiles of chlamydial proteins from the lysate of infected cells. In this way, CT621 was identified. CT621 is a member of a family of proteins containing a domain of unknown function DUF582 that is only found within the genus Chlamydia. Immunofluorescence microscopy and immunoblotting demonstrated that CT621 is secreted late in the chlamydial developmental cycle and that it is the first chlamydial protein found to be localized within both the host cell cytoplasm and the nucleus. To determine whether CT621 is secreted through the T3SS, an inhibitor of this apparatus was added to the infection medium, resulting in retention of the protein inside the chlamydiae. Hence, the so far uncharacterized CT621 is a new type III secretion effector protein.
Figures



Similar articles
-
Conserved type III secretion system exerts important roles in Chlamydia trachomatis.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5404-14. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337183 Free PMC article. Review.
-
Chlamydia trachomatis secretion of hypothetical protein CT622 into host cell cytoplasm via a secretion pathway that can be inhibited by the type III secretion system inhibitor compound 1.Microbiology (Reading). 2011 Apr;157(Pt 4):1134-1144. doi: 10.1099/mic.0.047746-0. Epub 2011 Jan 13. Microbiology (Reading). 2011. PMID: 21233161 Free PMC article.
-
The DUF582 Proteins of Chlamydia trachomatis Bind to Components of the ESCRT Machinery, Which Is Dispensable for Bacterial Growth In vitro.Front Cell Infect Microbiol. 2016 Oct 7;6:123. doi: 10.3389/fcimb.2016.00123. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27774439 Free PMC article.
-
Identification of a family of effectors secreted by the type III secretion system that are conserved in pathogenic Chlamydiae.Infect Immun. 2011 Feb;79(2):571-80. doi: 10.1128/IAI.00825-10. Epub 2010 Nov 15. Infect Immun. 2011. PMID: 21078856 Free PMC article.
-
Got mutants? How advances in chlamydial genetics have furthered the study of effector proteins.Pathog Dis. 2021 Feb 4;79(2):ftaa078. doi: 10.1093/femspd/ftaa078. Pathog Dis. 2021. PMID: 33512479 Free PMC article. Review.
Cited by
-
CteG is a Chlamydia trachomatis effector protein that associates with the Golgi complex of infected host cells.Sci Rep. 2019 Apr 16;9(1):6133. doi: 10.1038/s41598-019-42647-3. Sci Rep. 2019. PMID: 30992493 Free PMC article.
-
Mapping immunodominant antigens and H-2-linked antibody responses in mice urogenitally infected with Chlamydia muridarum.Microbes Infect. 2012 Jul;14(7-8):659-65. doi: 10.1016/j.micinf.2012.02.005. Epub 2012 Mar 3. Microbes Infect. 2012. PMID: 22421110 Free PMC article.
-
Secretion of the chlamydial virulence factor CPAF requires the Sec-dependent pathway.Microbiology (Reading). 2010 Oct;156(Pt 10):3031-3040. doi: 10.1099/mic.0.040527-0. Epub 2010 Jun 3. Microbiology (Reading). 2010. PMID: 20522495 Free PMC article.
-
Conserved type III secretion system exerts important roles in Chlamydia trachomatis.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5404-14. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337183 Free PMC article. Review.
-
Sigma 54-Regulated Transcription Is Associated with Membrane Reorganization and Type III Secretion Effectors during Conversion to Infectious Forms of Chlamydia trachomatis.mBio. 2020 Sep 8;11(5):e01725-20. doi: 10.1128/mBio.01725-20. mBio. 2020. PMID: 32900805 Free PMC article.
References
-
- Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol Rev. 2005;29:949–959. - PubMed
-
- Bailey L, Gylfe A, Sundin C, et al. Small molecule inhibitors of type III secretion in Yersinia block the Chlamydia pneumoniae infection cycle. FEBS Lett. 2007;581:587–595. - PubMed
-
- Bavoil PM, Hsia RC. Type III secretion in Chlamydia: a case of deja vu? Mol Microbiol. 1998;28:860–862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
