Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function
- PMID: 19682204
- PMCID: PMC2773444
- DOI: 10.1111/j.1471-4159.2009.06335.x
Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function
Abstract
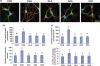
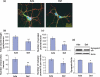
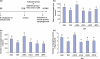
Docosahexaenoic acid (DHA, 22:6n-3), the major polyunsaturated fatty acid accumulated in the brain during development, has been implicated in learning and memory, but underlying cellular mechanisms are not clearly understood. Here, we demonstrate that DHA significantly affects hippocampal neuronal development and synaptic function in developing hippocampi. In embryonic neuronal cultures, DHA supplementation uniquely promoted neurite growth, synapsin puncta formation and synaptic protein expression, particularly synapsins and glutamate receptors. In DHA-supplemented neurons, spontaneous synaptic activity was significantly increased, mostly because of enhanced glutamatergic synaptic activity. Conversely, hippocampal neurons from DHA-depleted fetuses showed inhibited neurite growth and synaptogenesis. Furthermore, n-3 fatty acid deprivation during development resulted in marked decreases of synapsins and glutamate receptor subunits in the hippocampi of 18-day-old pups with concomitant impairment of long-term potentiation, a cellular mechanism underlying learning and memory. While levels of synapsins and NMDA receptor subunit NR2A were decreased in most hippocampal regions, NR2A expression was particularly reduced in CA3, suggesting possible role of DHA in CA3-NMDA receptor-dependent learning and memory processes. The DHA-induced neurite growth, synaptogenesis, synapsin, and glutamate receptor expression, and glutamatergic synaptic function may represent important cellular aspects supporting the hippocampus-related cognitive function improved by DHA.
Figures



References
-
- Aarnisalo P, Kim CH, Lee JW, Perlmann T. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. J. Biol. Chem. 2002;277:35118–35123. - PubMed
-
- Ahmad A, Moriguchi T, Salem N., Jr Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr. Neurol. 2002;26:210–218. - PubMed
-
- Birch EE, Garfield S, Hoffman DR, Uauy RD, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000;42:174–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

