A helical membrane-binding domain targets the Toxoplasma ROP2 family to the parasitophorous vacuole
- PMID: 19682324
- PMCID: PMC2746882
- DOI: 10.1111/j.1600-0854.2009.00958.x
A helical membrane-binding domain targets the Toxoplasma ROP2 family to the parasitophorous vacuole
Abstract
During invasion, the obligate intracellular pathogen, Toxoplasma gondii, secretes into its host cell a variety of effector molecules, several of which have been implicated in strain-specific variation in disease. The largest family of these effectors, defined by the canonical member ROP2, quickly associates with the nascent parasitophorous vacuole membrane (PVM) after secretion. Here we demonstrate that the NH(2)-terminal domain of the ROP2 family contains a series of amphipathic helices that are necessary and sufficient for membrane association. While each of the amphipathic helices is individually competent to bind cellular membranes, together they act to bind the PVM preferentially, possibly through sensing its strong negative curvature. This previously uncharacterized helical domain is an evolutionarily robust and energetically efficient design for membrane association.
Figures

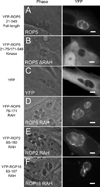
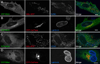
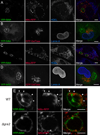

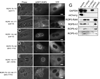



Similar articles
-
The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane.J Cell Biol. 2001 Jul 9;154(1):95-108. doi: 10.1083/jcb.200101073. J Cell Biol. 2001. PMID: 11448993 Free PMC article.
-
Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane.Cell Microbiol. 2007 Jan;9(1):54-64. doi: 10.1111/j.1462-5822.2006.00767.x. Epub 2006 Jul 31. Cell Microbiol. 2007. PMID: 16879455
-
Proximity-Labeling Reveals Novel Host and Parasite Proteins at the Toxoplasma Parasitophorous Vacuole Membrane.mBio. 2021 Dec 21;12(6):e0026021. doi: 10.1128/mBio.00260-21. Epub 2021 Nov 9. mBio. 2021. PMID: 34749525 Free PMC article.
-
The Toxoplasma gondii parasitophorous vacuole membrane: transactions across the border.J Eukaryot Microbiol. 2007 Jan-Feb;54(1):25-8. doi: 10.1111/j.1550-7408.2006.00230.x. J Eukaryot Microbiol. 2007. PMID: 17300514 Review.
-
Toxoplasma Mechanisms for Delivery of Proteins and Uptake of Nutrients Across the Host-Pathogen Interface.Annu Rev Microbiol. 2020 Sep 8;74:567-586. doi: 10.1146/annurev-micro-011720-122318. Epub 2020 Jul 17. Annu Rev Microbiol. 2020. PMID: 32680452 Free PMC article. Review.
Cited by
-
Rhoptry and Dense Granule Secreted Effectors Regulate CD8+ T Cell Recognition of Toxoplasma gondii Infected Host Cells.Front Immunol. 2019 Sep 6;10:2104. doi: 10.3389/fimmu.2019.02104. eCollection 2019. Front Immunol. 2019. PMID: 31555296 Free PMC article.
-
Toxoplasma gondii ROP17 inhibits the innate immune response of HEK293T cells to promote its survival.Parasitol Res. 2019 Mar;118(3):783-792. doi: 10.1007/s00436-019-06215-y. Epub 2019 Jan 23. Parasitol Res. 2019. PMID: 30675671
-
Secretion of Rhoptry and Dense Granule Effector Proteins by Nonreplicating Toxoplasma gondii Uracil Auxotrophs Controls the Development of Antitumor Immunity.PLoS Genet. 2016 Jul 22;12(7):e1006189. doi: 10.1371/journal.pgen.1006189. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27447180 Free PMC article.
-
Functional Analysis of the Rhoptry Kinome during Chronic Toxoplasma gondii Infection.mBio. 2016 Jun 14;7(3):e00842-16. doi: 10.1128/mBio.00842-16. mBio. 2016. PMID: 27302762 Free PMC article.
-
Association of host mitochondria with the parasitophorous vacuole during Toxoplasma infection is not dependent on rhoptry proteins ROP2/8.Int J Parasitol. 2010 Oct;40(12):1367-71. doi: 10.1016/j.ijpara.2010.07.002. Epub 2010 Jul 15. Int J Parasitol. 2010. PMID: 20637758 Free PMC article.
References
-
- Baekkeskov S, Kanaani J. Palmitoylation cycles and regulation of protein function (Review) Mol Membr Biol. 2009:1–13. - PubMed
-
- Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9(2):99–111. - PubMed
-
- Charron AJ, Sibley LD. Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic. 2004;5(11):855–867. - PubMed
-
- Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6(1):79–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

