Understanding loss of donor white blood cell immunogenicity after pathogen reduction: mechanisms of action in ultraviolet illumination and riboflavin treatment
- PMID: 19682337
- PMCID: PMC2865145
- DOI: 10.1111/j.1537-2995.2009.02333.x
Understanding loss of donor white blood cell immunogenicity after pathogen reduction: mechanisms of action in ultraviolet illumination and riboflavin treatment
Abstract
Background: Donor white blood cells (WBCs) present in transfusion products can lead to immune sequelae such as production of HLA antibodies or graft-versus-host disease in susceptible transfusion recipients. Eliminating the immunogenicity of blood products may prove to be of clinical benefit, particularly in patients requiring multiple transfusions in whom allosensitization is common. This study examines a method of pathogen reduction based on ultraviolet light illumination in the presence of riboflavin. In addition to pathogens, WBCs treated with this system are affected and fail to stimulate proliferation of allogeneic peripheral blood mononuclear cells (PBMNCs) in vitro.
Study design and methods: This study sought to determine the mechanisms regulating this loss of immunogenicity. Treated cells were examined for surface expression of a number of molecules involved in activation and adhesion, viability, cell-cell conjugation, and ability to stimulate immune responses in allogeneic PBMNCs.
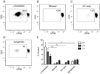
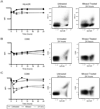
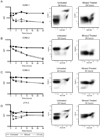
Results: Compared with untreated controls, ultraviolet (UV)-irradiated antigen-presenting cells showed slightly reduced surface expression of HLA Class II and costimulatory molecules and had more significant reductions in surface expression of a number of adhesion molecules. Furthermore, treated cells had a severe defect in cell-cell conjugation. The observed loss of immunogenicity was nearly complete, with UV-irradiated cells stimulating barely measurable interferon-gamma production and no detectable STAT-3, STAT-5, or CD3-epsilon phosphorylation in allospecific primed T cells.
Conclusion: These results suggest that defective cell-cell adhesion prevents UV-irradiated cells from inducing T-cell activation.
Conflict of interest statement
Conflict of Interest: Rachael P. Jackman and John W. Heitman have no conflict of interest. Susanne Marschner and Raymond P. Goodrich are employed by CaridianBCT Biotechnologies, Philip J. Norris has a consulting relationship with and received research funding for this project from CaridianBCT Biotechnologies.
Figures



References
-
- Goodrich RP, Edrich RA, Li J, Seghatchian J. The Mirasol PRT system for pathogen reduction of platelets and plasma: an overview of current status and future trends. Transfus Apher Sci. 2006;35:5–17. - PubMed
-
- Cardo LJ, Rentas FJ, Ketchum L, Salata J, Harman R, Melvin W, Weina PJ, Mendez J, Reddy H, Goodrich R. Pathogen inactivation of Leishmania donovani infantum in plasma and platelet concentrates using riboflavin and ultraviolet light. Vox Sang. 2006;90:85–91. - PubMed
-
- Cardo LJ, Salata J, Mendez J, Reddy H, Goodrich R. Pathogen inactivation of Trypanosoma cruzi in plasma and platelet concentrates using riboflavin and ultraviolet light. Transfus Apher Sci. 2007;37:131–137. - PubMed
-
- Rentas F, Harman R, Gomez C, Salata J, Childs J, Silva T, Lippert L, Montgomery J, Richards A, Chan C, Jiang J, Reddy H, Li J, Goodrich R. Inactivation of Orientia tsutsugamushi in red blood cells, plasma, and platelets with riboflavin and light, as demonstrated in an animal model. Transfusion. 2007;47:240–247. - PubMed
-
- Fast LD, Dileone G, Li J, Goodrich R. Functional inactivation of white blood cells by Mirasol treatment. Transfusion. 2006;46:642–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

