Cellular ADMA: regulation and action
- PMID: 19682580
- PMCID: PMC2767414
- DOI: 10.1016/j.phrs.2009.08.002
Cellular ADMA: regulation and action
Abstract
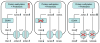
Asymmetric (N(G),N(G)) dimethylarginine (ADMA) is present in plasma and cells. It can inhibit nitric oxide synthase (NOS) that generates nitric oxide (NO) and cationic amino acid transporters (CATs) that supply intracellular NOS with its substrate, l-arginine, from the plasma. Therefore, ADMA and its transport mechanisms are strategically placed to regulate endothelial function. This could have considerable clinical impact since endothelial dysfunction has been detected at the origin of hypertension and chronic kidney disease (CKD) in human subjects and may be a harbinger of large vessel disease and cardiovascular disease (CVD). Indeed, plasma levels of ADMA are increased in many studies of patients at risk for, or with overt CKD or CVD. However, the levels of ADMA measured in plasma of about 0.5micromol.l(-1) may be below those required to inhibit NOS whose substrate, l-arginine, is present in concentrations many fold above the Km for NOS. However, NOS activity may be partially inhibited by cellular ADMA. Therefore, the cellular production of ADMA by protein arginine methyltransferase (PRMT) and protein hydrolysis, its degradation by N(G),N(G)-dimethylarginine dimethylaminohydrolase (DDAH) and its transmembrane transport by CAT that determines intracellular levels of ADMA may also determine the state of activation of NOS. This is the focus of the review. It is concluded that cellular levels of ADMA can be 5- to 20-fold above those in plasma and in a range that could tonically inhibit NOS. The relative importance of PRMT, DDAH and CAT for determining the intracellular NOS substrate:inhibitor ratio (l-arginine:ADMA) may vary according to the pathophysiologic circumstance. An understanding of this important balance requires knowledge of these three processes that regulate the intracellular levels of ADMA and arginine.
Conflict of interest statement
None.
Figures

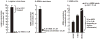

References
-
- Ogawa T, Kimoto M, Sasaoka K. Occurrence of a new enzyme catalyzing the direct conversion of NG,NG-dimethyl-L-arginine to L-citrulline in rats. Biochem Biophys Res Commun. 1987;148:671–7. - PubMed
-
- Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20:S60–S62. - PubMed
-
- Ueda S, Kato S, Matsuoka H, Kimoto M, Okuda S, Morimatsu M, et al. Regulation of cytokine induced nitric oxide synthasis by asymmetric dimethylarginine. Circ Res. 2003;92:226–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

